
Vascular aging in adult congenital heart disease-a narrative review
Introduction
Owing to advances in surgical procedures and medical treatments, patients with congenital heart disease (CHD) have a long life expectancy (1). Therefore, the influence of the aging process needs to be considered in the management of patients. Systemic blood pressure gradually elevates with age, and high blood pressure can cause potentially fatal conditions such as ischemic heart disease, cerebral vascular disease, and renal failure. High blood pressure is common in patients with CHD (2) and cardiovascular disease (e.g., ischemic heart disease, cerebrovascular disease, and renal disease) is common in adults with CHD (3-6), according to previous research. Their hearts are vulnerable to the effects of high blood pressure because they have been overloaded in some form since birth (e.g., cyanosis, volume overload, and systemic right ventricle). High blood pressure can have a detrimental effect on their cardiovascular health. The cause of hypertension in CHD patients is unknown; however, renal factors may play a role, given that cyanosis and cardiac surgery can both impair renal function (7).
A man is said to be as old as his arteries. One of the key factors in hypertension and cardiovascular disease is vascular aging. Early vascular aging (EVA), which refers to the acceleration of the aging process of the elastic arteries, has recently emerged as a promising tool for clinical guidance in the treatment of people who are at risk for cardiovascular disease (8,9). In the definition of the EVA, the stiffness of large arteries is generally evaluated and referenced. The enhancement of aortic stiffness impairs the reservoir function of large arteries.
Concerning large arteries in CHD, morphological evaluation is commonly performed. However, there is not much research targeting reservoir function of large arteries in patients with CHD. Aortopathy, a common condition in CHD, is a term used to describe structural abnormalities of the aorta in patients with CHD. The aortopathy in CHD patients is similar to that of aged aorta from a histological standpoint. In fact, we previously reported that the prevalence of EVA is high in CHD patients (10).
In this article, I will review the studies on systemic vascular physiology in patients with CHD and discuss vascular aging. I present the following article in accordance with the Narrative Review reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-218/rc).
Methods
Relevant studies published from January 1, 1973 to June 30, 2022 were identified via a PubMed search using different combinations of the following search terms: “early vascular aging”, “vascular aging”, “congenital heart disease”, “pulse wave velocity”, “aortic coarctation”, “tetralogy of Fallot”, “hypoplastic left heart syndrome”, “pressure wave reflection”, “subendocardial viability ratio”, “arterial stiffness gradient”. Most of the search formulas I use are shown in Table 1. Additional papers were identified by reviewing reference lists of relevant publications. Publications with relative low credibility and non-English publications were excluded. Data were extracted based on their relevance to the topic instead of implementing a systematic approach to paper selection. More details of the method are shown in Table 2.
Table 1
| “early vascular aging” |
| “vascular aging” AND “congenital heart disease” |
| “pulse wave velocity” AND “congenital heart disease” |
| “pulse wave velocity” AND “aortic coarctation” |
| “pulse wave velocity” AND “tetralogy of Fallot” |
| “pulse wave velocity” AND “hypoplastic left heart syndrome” |
| “pressure wave reflection” AND “congenital heart disease” |
| “pressure wave reflection” AND “aortic coarctation” |
| “pressure wave reflection” AND “tetralogy of Fallot” |
| “pressure wave reflection” AND “hypoplastic left heart syndrome” |
| “subendocardial viability ratio” AND “congenital heart disease” |
| “aortic stiffness gradient” |
Table 2
| Items | Specification |
|---|---|
| Date of search (specified to date, month and year) | 2022/2/9–2022/6/30 |
| Databases and other sources searched | PubMed |
| Search terms used (including MeSH and free text search terms and filters) | See Table 1 for details |
| Timeframe | 1973–2022 |
| Inclusion and exclusion criteria (study type, language restrictions etc.) | Inclusion criteria: research articles and reviews in English about themes. Exclusion criteria: some papers which I considered with low reliability |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | TM conducted the selection |
| Any additional considerations, if applicable | Some papers were identified by reviewing reference lists of relevant publications |
What is vascular aging?
It is well known that the aorta stiffens with age. The histological changes of aortic aging consist of elastin fracture, collagen storage, and calcium deposition (11). The elastin fracture progresses with a large strain and cyclic number (12). Moreover, metabolic factors, such as advanced glycation end products, accelerate the stiffening of the aorta (13). These histological changes of the aorta with age are similar to the so-called aortopathy in CHD (14).
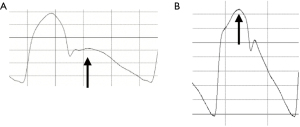
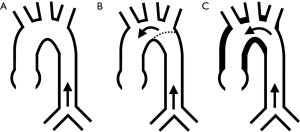
The functional markers of aortic aging are elevation of pulse wave velocity (PWV) and damaged aortic reservoir function. The elevation of PWV results in an early return of reflected pressure to the heart (15). Figure 1 shows ascending aortic pressure waveforms in young (Figure 1A) and old (Figure 1B). Because of the low PWV in adolescents, the reflected pressure wave (arrow) returns to the heart during diastole (Figure 1A). However, PWV elevates with age, and the reflected pressure (arrow) wave returns to the heart during systole (Figure 1B), increasing left ventricular afterload. Furthermore, because the reflected pressure wave returns to the heart early, the forward and reflected pressure have a small time phase difference, resulting in a large central pulse pressure (so-called isolated systolic hypertension, which is common in elderly people). Concerning aortic reservoir function, more than half of the blood ejected from the left ventricle is temporarily stored in the aorta during systole and then runs off during diastole (16). The heart is one of the most important organs that is primarily perfused during diastole (coronary circulation). The reflected pressure wave that returns to the heart during diastole can help to increase the amount of blood that is stored during systole and runs off to the heart during diastole. However, as the aorta loses elasticity with age, its role as a functional reservoir is diminished, because the reflected pressure wave returns to the heart during systole due to the increased PWV.
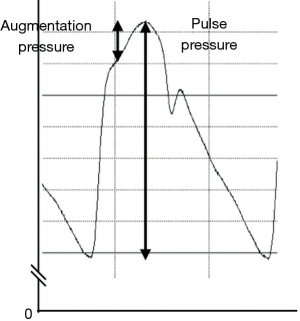
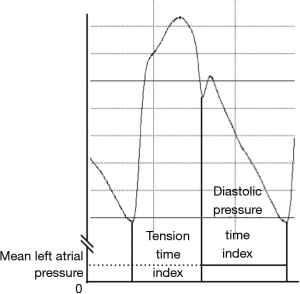
The PWV value is used as a parameter of EVA. EVA is defined as PWV values higher than the 95th percentile for age and sex (17). One of the reasons why PWV is generally used in the evaluation of vascular aging is its convenience of measurement. As mentioned above, the increment of PWV becomes disadvantageous to systemic circulation through the increment of pressure wave reflection. Therefore, the augmentation index, which is a commonly used measurement in evaluating pressure wave reflection (18), can be important in the evaluation of vascular aging. The augmentation index is defined as the ratio of augmentation pressure to pulse pressure, and the index becomes negative when the peak systolic pressure precedes the inflection point (Figure 2). The augmentation index is important parameter in evaluating aortic reservoir function, however, the measurement of the augmentation index has technical difficulties (identification of inflection point) compared to PWV measurement. It may be one of the reasons why the PWV is commonly used in clinical practice. Regarding the aortic reservoir function, subendocardial viability ratio is commonly adopted for evaluation (19,20) and can be one of the important biomarkers in evaluating vascular aging. The measurement of the subendocardial viability ratio is also more complex than the measurement of PWV (Figure 3).
Vascular aging in CHD
PWV in patients with CHD
Although we previously reported that EVA is common in adults with CHD (10), it means their PWV is elevated. Some studies reported PWV in patients with various types of CHD, especially aortic arch disease, tetralogy of Fallot (TOF), Fontan candidates, and the results of measurements were varied. Harteveld et al. (21) demonstrated an increase in carotid-femoral PWV in patients following Fontan surgery. However, Sarkola’s group (22) and Tomkiewics-Pajak’s group (23) reported that the carotid-femoral PWV values in patients with Fontan circulation were not significantly different from the data in control subjects. Concerning patients with repaired TOF, which is the most common cyanotic heart disease, Cheung and colleagues demonstrated that the heart-femoral PWV in the patients is higher than that in control subjects (666±15 vs. 587±81 cm/s, P=0.021) (24). However, there was no difference in carotid-femoral PWV between patients with repaired TOF and controls in de Groot’s study (25). In addition, Currie and colleagues also reported that whole-body PWV (heart-dorsal pedal artery in the study) was not elevated in children with TOF and aortic coarctation (26). Tronjnarska and colleagues reported that carotid-femoral PWV in adult cyanotic patients with CHD was elevated (7.40±2.07 vs. 6.33±0.76 m/s, P=0.003) (27). Weismann et al. (28) reported that the carotid-femoral PWV was not elevated in patients with bicuspid aortic valve, one of the most common CHDs. The age of the patients could be one of the reasons for the wide variety of PWV values. In our study of adults with CHD (10), we found that the prevalence of EVA was high in elderly CHD patients. The age of the patients enrolled in each study may have an influence on the result. Another reason could be the range of the examined artery (see below).
Pressure wave reflection in patients with CHD
The degree of enhancement of pressure wave reflection is typically measured using the augmentation index (18) (Figure 2). We previously reported that adults with CHD commonly have a high augmentation index (29). Many studies have shown that patients with Fontan circulation [(17.5±9.9)% vs. (11.2±7.8)%, P=0.006] (21), [(17.01±3.3)% vs. (6.05±11.0)%, P<0.001] (23), cyanosis [(24.75±13.49)% vs. (3.03±13.57)%, P=0.00001] (27), bicuspid aortic valve (10.1% vs. 6.3%, P<0.001) and TOF [(−14.1±17.0)% vs. (−25.2±14.6)%, P=0.016] (24) have an increased augmentation index. From the theory mentioned above, the high PWV enhances pressure wave reflection. However, some reports demonstrated a high augmentation index without a high PWV (23,28). The prevalence of enhanced augmentation index in adults with CHD in our study (44.4%) (29) is higher than that of EVA (15.6%) (10). It is possible that pressure wave reflection in CHD patients is enhanced through a mechanism other than PWV enhancement.
Aortic reservoir function in patients with CHD
The subendocardial viability ratio (Figure 3) is an important biomarker or aortic reservoir function. In Figure 1, the subendocardial viability ratio of the pressure waveform A is higher than that in the pressure waveform B. We previously measured the subendocardial viability ratio in pediatric patients with repaired aortic coarctation (30), repaired TOF (31) and transposition of great arteries after arterial switch operation (32). In comparison with age-matched controls, the subendocardial viability ratio was preserved in each study. The ratio can be preferentially maintained because it represents the balance of cardiac workload and blood supply to the heart. The patients in each study did not have heart failure, so the subendocardial viability ratio could be maintained. Saiki and colleagues reported a low subendocardial viability ratio in Norwood procedure patients, and the low value was associated with poor outcomes (33).
Regional PWV in patients with CHD
Recently, studies concerning reginal PWV have been increasing. One of the reasons increasing the studies may be progress of magnetic resonance imaging. Those papers reported that the PWV in the proximal aorta in patients with aortic coarctation (34,35) and TOF (36,37) was higher than that in control subjects, although there were variations in the measurement position. Some papers reported an increase in PWV in the proximal aorta (38,39) in patients with Fontan circulation (mostly hypoplastic left heart syndrome patients), while others reported no increase (40,41).
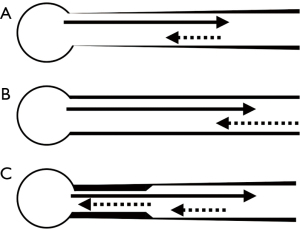
In some studies, researchers measured the PWV at the proximal and distal aorta, although there were variations in the measurement sites (Table 3). The PWV in the whole aorta was not necessarily elevated (see above). However, these data concerning the regional PWV suggest that the PWV in the proximal aorta elevated in patients with CHD. Another notable thing is the arterial stiffness gradient (42). In control subjects with a normal arterial tree, the PWV in the proximal aorta was smaller than that in the distal aorta. However, in CHD patients (Table 3), the enhancement of the PWV in the proximal aorta results in the discontinuity of the stiffness gradient. In normal vascular tree, stiffness gradient, which means a constant increase in stiffness from the aorta to the periphery, is formed by the loss of elastin content and the decrease in vessel diameter (Figure 4A) (42). By the study in aortic aging, the importance of “normal” stiffness gradient has been recently come to light. Age-related increase in aortic stiffness causes the loss of stiffness gradient and results in increasing forward wave amplitude and pulse pressure (Figure 4B) (43). The loss of stiffness gradient leads to the transmission of a highly pulsatile pressure wave into the microcirculation (44). It can lead to endothelial dysfunction, reducing organ perfusion, and ultimately organ dysfunction (45). A recent study in a dialysis cohort has shown that the aortic-brachial arterial stiffness mismatch was strongly associated with increased mortality (46). The elevated proximal aortic stiffness causes a distal shift in major reflection sites and a paradoxical increase in reflected wave amplitude, resulting in a dissociation between aortic stiffness and augmentation index (47). The discontinuity of stiffness gradient in the CHD patients is different from the loss of stiffness gradient in elderly people in that the aorta is stiff partially (Figure 4C). Moreover, the degree of the impedance mismatch in CHD patients (Table 3) is larger than that in aging process (42). Therefore, the impedance mismatch in CHD patients can elevate augmentation index and cause disadvantage for microcirculation. The influence of the discontinuity of stiffness gradient on hemodynamics in CHD patients is more complicated than that of the loss stiffness gradient in the aged individuals.
Table 3
| Study | Objects | PWV | |
|---|---|---|---|
| Proximal | Distal | ||
| Schäfer et al. (38) | HLHS | 5.1 m/s* | 3.1 m/s |
| Control | 2.6 m/s | 2.7 m/s | |
| Voges et al. (40) | HLHS | 3.7±1.4 m/s | 3.7±1.3 m/s |
| Control | 3.2±0.4 m/s | 4.0±1.0 m/s | |
| Voges et al. (34) | CoA | 4.6±1.7 m/s* | 4.3±1.6 m/s |
| Control | 3.5±0.8 m/s | 3.9±0.8 m/s | |
| Cheung et al. (24) | TOF | 666±151 cm/s* | Right: 888±202 cm/s, left: 918±207 cm/s |
| Control | 587±81 cm/s | Right: 845±207 cm/s, left: 851±215 cm/s | |
| Saiki et al. (37) | Repaired TOF | 588±205 cm/s* | 441±189 cm/s |
| Unrepaired TOF | 680±288 cm/s* | 430±114 cm/s | |
| Control | 439±101 cm/s | 461±164 cm/s | |
Values are presented as mean ± standard deviation. *, P<0.05 vs. control. PWV, pulse wave velocity; HLHS, hypoplastic left heart syndrome; CoA, coarctation of the aorta; TOF, tetralogy of Fallot.
One of the possible mechanisms of EVA in CHD—the enhancement of pressure wave reflection
In patients with CHD, surgical management and hemodynamics can influence the aortic aging process and induce EVA. Judging from the study concerning pressure wave reflection (29).
Despite apparently successful surgical repair of aortic coarctations, the early onset of cardiovascular diseases (hypertension, myocardial infarction, cardiac failure, and sudden death) has been frequently encountered (48-50). One of the possible mechanisms of it is the enhancement of pressure wave reflection (51). It is well known that the distensibility of the reconstructed site in patients after a repair of coarctation is decreased (52,53). Wave reflections arise from any discontinuity in which there is a change in impedance (18). Therefore, the reconstructed aortic site could generate a reflection wave that traveled backward to the ascending aorta. Because the repaired site is nearer to the heart than the original reflection site (region of the main aortic bifurcation) (Figure 5) (54), the influence of pressure wave reflection becomes strong. As a result, central blood pressure can elevate and cardiovascular disease increases (Figure 6).
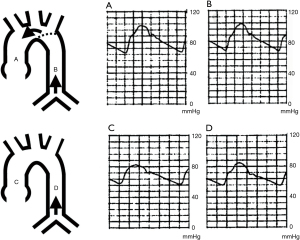
A systemic-pulmonary shunt operation is commonly performed in patients with cyanotic CHD to improve pulmonary blood flow and reduce cyanosis. This procedure increases ascending aortic blood flow without affecting descending aortic blood flow. The elastin fracture progresses with a large strain and cyclic number (12). Therefore, the regional increment of blood flow (which means an increment of strain) induces a regional increment of elastin fracture and results in a regional increment of PWV. As a result, the aorta can have a discontinuity in stiffness gradient, and the condition can make a new reflected pressure wave (Figure 5). Collateral vessels from the systemic arterial tree to the pulmonary arterial system frequently develop in patients with cyanotic CHD who do not have a systemic-pulmonary shunt. These vessels can function similarly to a systemic-pulmonary shunt in patients with cyanotic heart disease, causing discontinuity of the arterial stiffness gradient.
Studies concerning vascular physiology in patients with CHD are still few. Further research is needed to improve long-term prognosis in CHD patients.
Conclusions
Various hemodynamics unique to CHD can cause the discontinuity of the stiffness gradient in the systemic arterial tree. Although it has been reported that cardiovascular disease is common in not only complex but also simple CHD (6), the discontinuity of the stiffness gradient must be one of the causes of hypertension and cardiovascular disease. It can cause cardiovascular disease and organ damage. Magnetic resonance imaging is one of the non-invasive methods evaluating the stiffness gradient of the aorta. The PWV is most used tool evaluating the vascular aging, however, it is insufficient in evaluation of systemic arterial system in CHD patients. The evaluation of pressure wave reflection may be superior to that of PWV in evaluating arterial system of them. However, it is more complex and also incomplete. Further research concerning the arterial system in patients with CHD is needed to find a valid evaluation method for their arterial system and improve the prognosis of patients with CHD.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Harald Kaemmerer, Yskert von Kodolitsch and Koichiro Niwa), for the series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part V” published in Cardiovascular Diagnosis and Therapy. The article has undergone external peer review.
Reporting Checklist: The author has completed the Narrative Review reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-218/rc
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-218/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-218/coif). The series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part V” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shiina Y, Toyoda T, Kawasoe Y, et al. Prevalence of adult patients with congenital heart disease in Japan. Int J Cardiol 2011;146:13-6. [Crossref] [PubMed]
- Murakami T, Horibata Y, Tateno S, et al. Blood pressure in adults with congenital heart disease. Vasc Fail 2021;4:39-45. [Crossref]
- Wang T, Chen L, Yang T, et al. Congenital Heart Disease and Risk of Cardiovascular Disease: A Meta-Analysis of Cohort Studies. J Am Heart Assoc 2019;8:e012030. [Crossref] [PubMed]
- Saha P, Potiny P, Rigdon J, et al. Substantial Cardiovascular Morbidity in Adults With Lower-Complexity Congenital Heart Disease. Circulation 2019;139:1889-99. [Crossref] [PubMed]
- Bauer UMM, Körten MA, Diller GP, et al. Cardiovascular risk factors in adults with congenital heart defects – Recognised but not treated? An analysis of the German National Register for Congenital Heart Defects. Int J Cardiol 2019;277:79-84. [Crossref] [PubMed]
- Videbæk J, Laursen HB, Olsen M, et al. Long-Term Nationwide Follow-Up Study of Simple Congenital Heart Disease Diagnosed in Otherwise Healthy Children. Circulation 2016;133:474-83. [Crossref] [PubMed]
- Greenberg JH, Zappitelli M, Devarajan P, et al. Kidney Outcomes 5 Years After Pediatric Cardiac Surgery: The TRIBE-AKI Study. JAMA Pediatr 2016;170:1071-8. [Crossref] [PubMed]
- Nilsson PM. Early vascular aging (EVA): consequences and prevention. Vasc Health Risk Manag 2008;4:547-52. [Crossref] [PubMed]
- Nilsson PM. Hemodynamic Aging as the Consequence of Structural Changes Associated with Early Vascular Aging (EVA). Aging Dis 2014;5:109-13. [Crossref] [PubMed]
- Murakami T, Horibata Y, Tateno S, et al. Early vascular aging in adult patients with congenital heart disease. Hypertens Res 2021;44:1122-8. [Crossref] [PubMed]
- Lakatta EG, Mitchell JH, Pomerance A, et al. Human aging: changes in structure and function. J Am Coll Cardiol 1987;10:42A-7A. [Crossref] [PubMed]
- Nichols WW, O’Rourke MF, Vlachopoulos C. editors. Aging. McDonald’s Blood Flow in Arteries 6th ed. London: Hodder Arnold, 2011:411-46.
- Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens 2003;21:3-12. [Crossref] [PubMed]
- Niwa K, Perloff JK, Bhuta SM, et al. Structural abnormalities of great arterial walls in congenital heart disease: light and electron microscopic analyses. Circulation 2001;103:393-400. [Crossref] [PubMed]
- O’Rourke MF, Safar ME, Dzau V. The Cardiovascular Continuum extended: aging effects on the aorta and microvasculature. Vasc Med 2010;15:461-8. [Crossref] [PubMed]
- London GM, Guerin AP. Influence of arterial pulse and reflected waves on blood pressure and cardiac function. Am Heart J 1999;138:220-4. [Crossref] [PubMed]
- Kotsis V, Stabouli S, Karafillis I, et al. Early vascular aging and the role of central blood pressure. J Hypertens 2011;29:1847-53. [Crossref] [PubMed]
- Nichols WW, O’Rourke MF, Vlachopoulos C. editors. Wave reflection. McDonald’s Blood Flow in Arteries 6th ed. London: Hodder Arnold, 2011:195-223.
- Buckberg GD, Fixler DE, Archie JP, et al. Experimental subendocardial ischemia in dogs with normal coronary arteries. Circ Res 1972;30:67-81. [Crossref] [PubMed]
- Buckberg GD, Olinger GN, Mulder DG, et al. Depressed postoperative cardiac performance. J Thorac Cardiovasc Surg 1975;70:974-94. [Crossref] [PubMed]
- Harteveld LM, Blom NA, Terol Espinosa de Los Monteros C, et al. Determinants of exercise limitation in contemporary paediatric Fontan patients with an extra cardiac conduit. Int J Cardiol 2021;341:31-8. [Crossref] [PubMed]
- Sarkola T, Jaeggi E, Slorach C, et al. Assessment of vascular remodeling after the Fontan procedure using a novel very high resolution ultrasound method: arterial wall thinning and venous thickening in late follow-up. Heart Vessels 2013;28:66-75. [Crossref] [PubMed]
- Tomkiewicz-Pajak L, Dziedzic-Oleksy H, Pajak J, et al. Arterial stiffness in adult patients after Fontan procedure. Cardiovasc Ultrasound 2014;12:15. [Crossref] [PubMed]
- Cheung YF, Ou X, Wong SJ. Central and peripheral arterial stiffness in patients after surgical repair of tetralogy of Fallot: implications for aortic root dilatation. Heart 2006;92:1827-30. [Crossref] [PubMed]
- de Groot PC, Thijssen D, Binkhorst M, et al. Vascular function in children with repaired tetralogy of Fallot. Am J Cardiol 2010;106:851-5. [Crossref] [PubMed]
- Currie KD, Martin AA, Millar PJ, et al. Vascular and autonomic function in preschool-aged children with congenital heart disease. Congenit Heart Dis 2012;7:289-97. [Crossref] [PubMed]
- Trojnarska O, Szczepaniak-Chicheł L, Gabriel M, et al. Arterial stiffness and arterial function in adult cyanotic patients with congenital heart disease. J Cardiol 2017;70:62-7. [Crossref] [PubMed]
- Weismann CG, Ljungberg S, Åkesson A, et al. Multimodal Assessment of Vascular and Ventricular Function in Children and Adults With Bicuspid Aortic Valve Disease. Front Cardiovasc Med 2021;8:643900. [Crossref] [PubMed]
- Murakami T, Tateno S, Kawasoe Y, et al. Aortic surgery is one of the risk factors for enhancement of pressure wave reflection in adult patients with congenital heart disease. Int J Cardiol 2014;175:451-4. [Crossref] [PubMed]
- Murakami T, Takeda A. Preserved Cardiac Blood Supply-Workload Balance in Pediatric Patients After Aortic Arch Repair. Pediatr Cardiol 2018;39:294-8. [Crossref] [PubMed]
- Takei K, Murakami T, Takeda A. Implication of Aortic Root Dilation and Stiffening in Patients with Tetralogy of Fallot. Pediatr Cardiol 2018;39:1462-7. [Crossref] [PubMed]
- Murakami T, Takei K, Ueno M, et al. Aortic reservoir function after arterial switch operation in elementary school-aged children. Circ J 2008;72:1291-5. [Crossref] [PubMed]
- Saiki H, Kuwata S, Kurishima C, et al. Vulnerability of Coronary Circulation After Norwood Operation. Ann Thorac Surg 2016;101:1544-51. [Crossref] [PubMed]
- Voges I, Kees J, Jerosch-Herold M, et al. Aortic stiffening and its impact on left atrial volumes and function in patients after successful coarctation repair: a multiparametric cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 2016;18:56. [Crossref] [PubMed]
- Quail MA, Short R, Pandya B, et al. Abnormal Wave Reflections and Left Ventricular Hypertrophy Late After Coarctation of the Aorta Repair. Hypertension 2017;69:501-9. [Crossref] [PubMed]
- Seki M, Kurishima C, Kawasaki H, et al. Aortic stiffness and aortic dilatation in infants and children with tetralogy of Fallot before corrective surgery: evidence for intrinsically abnormal aortic mechanical property. Eur J Cardiothorac Surg 2012;41:277-82. [Crossref] [PubMed]
- Saiki H, Kojima T, Seki M, et al. Marked disparity in mechanical wall properties between ascending and descending aorta in patients with tetralogy of Fallot. Eur J Cardiothorac Surg 2012;41:570-3. [Crossref] [PubMed]
- Schäfer M, Younoszai A, Truong U, et al. Influence of aortic stiffness on ventricular function in patients with Fontan circulation. J Thorac Cardiovasc Surg 2019;157:699-707. [Crossref] [PubMed]
- Noortman LCM, Haapala EA, Takken T. Arterial Stiffness and Its Relationship to Cardiorespiratory Fitness in Children and Young Adults with a Fontan Circulation. Pediatr Cardiol 2019;40:784-91. [Crossref] [PubMed]
- Voges I, Jerosch-Herold M, Wegner P, et al. Frequent Dilatation of the Descending Aorta in Children With Hypoplastic Left Heart Syndrome Relates to Decreased Aortic Arch Elasticity. J Am Heart Assoc 2015;4:e002107. [Crossref] [PubMed]
- Voges I, Jerosch-Herold M, Hedderich J, et al. Maladaptive aortic properties in children after palliation of hypoplastic left heart syndrome assessed by cardiovascular magnetic resonance imaging. Circulation 2010;122:1068-76. [Crossref] [PubMed]
- Fortier C, Agharazii M. Arterial Stiffness Gradient. Pulse (Basel) 2016;3:159-66. [Crossref] [PubMed]
- Stone K, Fryer S, Meyer ML, et al. The aortic-femoral arterial stiffness gradient: an atherosclerosis risk in communities (ARIC) study. J Hypertens 2021;39:1370-7. [Crossref] [PubMed]
- Briet M, Boutouyrie P, Laurent S, et al. Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int 2012;82:388-400. [Crossref] [PubMed]
- Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol (1985) 2008;105:1652-60. [PubMed]
- Fortier C, Mac-Way F, Desmeules S, et al. Aortic-brachial stiffness mismatch and mortality in dialysis population. Hypertension 2015;65:378-84. [Crossref] [PubMed]
- Hickson SS, Nichols WW. Influence of the central-to-peripheral arterial stiffness gradient on the timing and amplitude of wave reflections. Hypertens Res 2016;39:723-9. [Crossref] [PubMed]
- Celermajer DS, Greaves K. Survivors of coarctation repair: fixed but not cured. Heart 2002;88:113-4. [Crossref] [PubMed]
- Toro-Salazar OH, Steinberger J, Thomas W, et al. Long-term follow-up of patients after coarctation of the aorta repair. Am J Cardiol 2002;89:541-7. [Crossref] [PubMed]
- O’Sullivan JJ, Derrick G, Darnell R. Prevalence of hypertension in children after early repair of coarctation of the aorta: a cohort study using casual and 24 hour blood pressure measurement. Heart 2002;88:163-6. [Crossref] [PubMed]
- Murakami T, Takeda A. Enhanced aortic pressure wave reflection in patients after repair of aortic coarctation. Ann Thorac Surg 2005;80:995-9. [Crossref] [PubMed]
- Xu J, Shiota T, Omoto R, et al. Intravascular ultrasound assessment of regional aortic wall stiffness, distensibility, and compliance in patients with coarctation of the aorta. Am Heart J 1997;134:93-8. [Crossref] [PubMed]
- Brili S, Dernellis J, Aggeli C, et al. Aortic elastic properties in patients with repaired coarctation of aorta. Am J Cardiol 1998;82:1140-3, A10.
- O’Rourke M. Arterial stiffness, systolic blood pressure, and logical treatment of arterial hypertension. Hypertension 1990;15:339-47. [Crossref] [PubMed]


