
Concomitant cardiovascular malformations in isolated bicuspid aortic valve disease: a retrospective cross-sectional study and meta-analysis
Introduction
The bicuspid aortic valve is the most frequent congenital heart defect in the general population. In echocardiographic and autopsy studies, the incidence of bicuspid aortic valve ranges from 0.16% in Asian (1) to 2% in the Western populations (2,3), and is more frequent in males (4). The abnormal architecture of the valve makes the leaflets susceptible to haemodynamic stress, leading to valvular thickening, calcification, and increased rigidity and narrowing of the aortic orifice (5). Independent of the cuspidity of the valve, women tend to present more often with moderate or severe aortic stenosis compared with men (4). Preventive measures, such as physical activity (6) and medicaments can’t decrease the risk of severe aortic valve stenosis, therefore aortic valve surgery is currently the only treatment to increase life expectancy and quality. Bicuspid aortic valve diagnosed in children often occurs in complex congenital heart defects or in syndromes such as Turner, Marfan, or Loeys-Dietz (7). In contrast, the coincidental finding of bicuspid aortic valves in healthy and asymptomatic adolescents and adults, frequently associated with dilation of the proximal aorta (8), are usually considered to occur as isolated cardiovascular manifestation. However, in such “isolated bicuspid aortic valve disease” the prevalence of other associated cardiovascular malformations has not been assessed systematically (9-11).
According to the literature, aortic coarctation may be the most common associated cardiovascular malformation in bicuspid aortic valve disease (12). More than half of the individuals with aortic coarctation (13), about 14% with anomalous coronary arteries (14), 8% with patent ductus arteriosus (15), and 8.5% with ventricular septal defects (16) had a concomitant bicuspid aortic valve. About 1% of adults with atrial septal defect (17) and 3% with mitral valve prolapse also had a bicuspid aortic valve (17,18), and even in individuals with tricuspid valve prolapse, a bicuspid aortic valve may be present simultaneously (19). However, there is no study that systematically reports imaging results for all associated cardiovascular malformations.
In our outpatient section for individuals with isolated bicuspid aortic valve, we routinely perform comprehensive imaging for associated cardiovascular malformations. Our first aim was to assess the prevalence of associated cardiovascular malformations such as aortic coarctation, coronary anomalies, patent ductus arteriosus, ventricular septal defect, atrial septal defect, mitral valve prolapse, and tricuspid valve prolapse in our study collective of 200 individuals, in a retrospective, observational manner. Our second aim was to assess the prevalence of these malformations in individuals assumed to have isolated bicuspid aortic valve disease by performing a systematic review of literature data followed by a meta-analysis. We applied STROBE as guideline for retrospective observational study quality (20) and PRISMA for meta-analysis (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-112/rc).
Methods
Individuals included in our retrospective study collective
We conducted a retrospective observational cross-sectional study of 200 consecutive individuals (154 males and 46 females), who presented to the adult cardiology outpatient department of the University Heart and Vascular Center in Hamburg with an isolated bicuspid aortic valve between January 2013 and December 2019. The indication for their visit comprised incidental finding of a bicuspid aortic valve for further clinical risk evaluation in 37%, symptomatic valve dysfunction including severe regurgitation or stenosis in 63%, and of these, indication for surgery for bicuspid aortic valve dysfunction or severe aneurysm in 40%. Individuals with complex cardiovascular malformations or with a known genetic aortic disease did not present to us as those are followed up in an adult congenital heart disease center or at a specialty consultation for genetic aortic disease. We collected anonymized patient data. According to German federal regulations, an approval for a retrospective study with anonymous data collection is not necessary. Our study complied with the Declaration of Helsinki (as revised in 2013). The study was approved by regional ethics committee of 2022_300194-WF.
Clinical manifestations in our retrospective study collective
We analyzed patient charts to assess age at first and final contact at our center. We documented the morphology and function of the aortic valve in all individuals. Morphologically, we distinguished bicuspid aortic valve type 1 with fusion of the right and the left coronary cusp; type 2 with right and non-coronary fusion; and type 3 with left and non-coronary fusion valves, according to Schaefer (21), or as “unknown” if not specified. We classified aortic valves exhibiting only one commissure as unicuspid. According to our clinical routine, we described aortic valve stenosis as at least moderate if the valve orifice area was less than 1.5 cm2 and considered regurgitation as at least moderate if the width of the vena contracta exceeded 3 mm, the pressure half time was below 500 ms or effective orifice area above 10 mm2 according to the current European echocardiography guidelines (22). We gathered information about surgery of the aortic valve and distinguished between aortic valve repair and aortic valve replacement with a biological or mechanical valve prosthesis, or pulmonary autograft known as Ross procedure (23).
In accordance with the current European Society of Cardiology guidelines for aortic disease, we considered the presence of root or ascending aortic dilation with diameters exceeding 40 mm (24) and described it as proximal aortic dilation. We measured aortic diameters by echocardiography using the end-diastolic leading-edge-to-leading-edge convention as this method showed accurate and reproducible values (25,26). Additionally, we assessed the aortic diameters using computed tomography or magnetic resonance imaging as recommended in current European Society of Cardiology guidelines for aortic disease (24).
Imaging methods used in our retrospective study collective
All 200 individuals underwent at least one transthoracic echocardiographic examination. In addition, 89 individuals underwent a transesophageal echocardiography. Indications for transesophageal echocardiography comprised evaluation of the aortic root anatomy prior to surgery or assessment of the severity of aortic valve dysfunction identified on transthoracic views. We performed tomographic examination of the aorta in 164 individuals, which comprised computed tomography in 34 individuals and magnetic resonance imaging tomography in 130 individuals. The tomographic imaging at baseline evaluation was aimed to map the entire aorta for aortic pathology including aneurysm, coarctation, or patent ductus arteriosus. We applied current diagnostic imaging criteria and technology as specified recently (27,28). Eighty-four individuals underwent coronary artery imaging with invasive coronary angiography performed in 75 and coronary computed tomography angiography in 10 individuals. Cardiac magnetic resonance imaging additionally was performed in 32 individuals. All examinations were performed to diagnose or rule out progressive coronary artery or ischemic heart disease for preoperative evaluation.
Identification of associated malformations in our retrospective study collective
We documented associated cardiovascular malformations according to charts as assessed during our clinical routine as follows:
Aortic coarctation, defined as local narrowing of the aortic lumen, based on reported findings on tomographic imaging (29), and in the case of two individuals based on description of surgical correction of aortic coarctation.
Coronary anomalies using at least one of the following imaging modalities for assessing coronary artery anatomy: coronary angiography, cardiac computed tomography, cardiac magnetic resonance. We described and included in our statistics any deviations from normal coronary anatomy as defined by Angelini (30).
Patent ductus arteriosus, a persistent vessel between aorta and pulmonary artery based on reported findings on tomographic imaging.
Atrial and ventricular septal defects with demonstration of a transseptal jet on color flow Doppler echocardiography. Small septal defects, such as patent foramen ovale, requiring contrast echocardiography, were excluded from of our statistical analysis.
Mitral valve and tricuspid valve morphology by echocardiography as stated by the guidelines of the European Association of Echocardiography (31,32) with criteria of mitral valve prolapse as described previously (32).
We calculated the competing risk for surgeries for associated malformations and for the bicuspid aortic disease. The group with surgeries for associated malformations included corrections of aortic coarctation, atrial and septal defects or mitral valve prolapse. The group of surgeries for the bicuspid aortic disease involved all surgeries on the proximal aorta, either for valve dysfunction, root or aorta ascendens aneurysms.
Meta-analysis of literature data
We performed a systematic review of the literature to assess published frequencies of malformations associated with non-syndromic bicuspid aortic valve disease (Figure 1). Two reviewers screened PubMed independently for the keyword “bicuspid aortic valve” up to 31 December 2019. We considered all studies published in English with inclusion of individuals of all ages with known bicuspid aortic valve. We excluded case reports, editorials, reviews, articles without abstracts or full text available online. We looked for the completeness of information about associated malformations and study population characteristics. We removed articles without information on concomitant malformation. The remaining articles included at least one of the following terms in the abstract or, if the abstract was not available, in the manuscript: aortic coarctation, ventricular septal defect, atrial septal defect, patent ductus arteriosus, mitral valve prolapse. We excluded reports with less than 5 individuals or with collectives that included predominantly individuals with syndromic diseases or complex malformation. Furthermore, we excluded reports not quantifying the frequency of the occurrence of concomitant malformations in a collective of individuals with bicuspid aortic valves. Two reviewers read thoroughly the studies appropriate for our meta-analysis and collected data on the frequency of malformations, primary imaging modalities and demographic parameters of the study group.
We assessed the risk of bias in individual studies by two independent raters. We graded the selection bias in the studies as low, intermediate, or high. Low, if the study population was close to the general population (for example asymptomatic individuals with incidental finding of a bicuspid aortic valve). On the other hand, we assumed a high bias in patient collectives presented in tertiary hospitals for surgeries. In case of different assessments of the two raters, we established a consensus.
Statistical methods
Unless otherwise specified, we expressed continuous data as means ± standard deviation and categorical data as absolute numbers with respective percentages in parentheses. A surgery performed on the aortic root, ascending aorta, or the valve itself was considered for statistical analysis as surgery for bicuspid aortic disease.
To compare the mean age at first surgery for associated malformations and at surgery on the proximal aorta including the aortic valve, we performed a t-test for independent samples. All tests were performed in an explorative manner, rather than testing a hypothesis postulated in advance, therefore we did not adjust for multiple testing.
Time to surgery (either associated malformations or proximal aorta) was performed with a competing risk survival analysis. In the meta-analysis we used a random effects model to compute the summary estimates.
The forest plots show the individual estimates and 95% confidence intervals, as well as of the summary estimates. We report I2 and τ2 as measures of heterogeneity. We used the statistics software R version 4.1.0 (34) for all statistical tests and plots, including the R-package survival version 3.2-3 (35) and R-package metafor version 2.4-0 (36).
Results
Baseline characteristics of individuals in our retrospective study collective
The mean age of individuals at initial contact at our center was 45±15 years (range, 14–80 years) and 77% were males. The most frequent bicuspid aortic valve morphology was type 1 according to Schaefer. Aortic valve surgery was performed in 100 individuals at 47±13 years (range, 21–75 years). At least moderate aortic valve dysfunction occurred in 132 (66.0%) individuals (Table 1). A total of 102 (51.0%) individuals had proximal aortic diameters exceeding 40 mm, of which 24 (12.0%) had aneurysms of the aortic root. We did not observe any statistically significant differences in aneurysm frequencies between the groups with different bicuspid aortic valve types. Aortic surgery was performed in 31.5% of these individuals at an age of 50±12 years (range, 21–77 years).
Table 1
| Variable | Number of individuals with findings (N=200), n (%) |
|---|---|
| Sex | |
| Male | 154 (77.0) |
| Female | 46 (23.0) |
| Bicuspid aortic valve morphology according to Schaefer | |
| Type 1 | 142 (71.0) |
| Type 2 | 35 (17.5) |
| Type 3 | 2 (1.0) |
| Unicuspid | 6 (3.0) |
| Unknown | 15 (7.5) |
| Aortic valve surgery performed, type | |
| No surgery | 100 (50.0) |
| Repair | 17 (8.5) |
| Biological valve | 64 (32.0) |
| Mechanical valve | 18 (9.0) |
| Ross procedure | 1 (0.5) |
| Aortic valve dysfunction (at least moderate) | |
| None | 68 (34.0) |
| Stenosis | 47 (23.5) |
| Regurgitation | 73 (36.5) |
| Stenosis and regurgitation | 12 (6.0) |
| Proximal aorta diameter >40 mm | 102 (51.0) |
| Proximal aortic surgery | 63 (31.5) |
Bicuspid aortic valve—associated malformations in our retrospective study collective
The most frequent malformation in our collective was coarctation of the aorta (Table 2). This associated malformation occurred in 7 (4.2%) of our individuals and was corrected in all individuals. Four individuals had type 1, two individuals type 2 bicuspid aortic valve, in one individual the valve type was not known. Surgical correction was performed at an age of 11±10 years (range, 4 months to 31 years). One individual underwent an initial correction at an age of 4 months and a second surgical correction at an age of 20 years. Coronary anomalies occurred in three (3.6%) individuals as an incidental finding. Two individuals had type 1, one individual type 2 bicuspid aortic valve. These anomalies were: persistent left superior vena cava draining into coronary sinus, high ostium of the left coronary artery plus persistent left superior vena cava draining into coronary sinus, and abnormal origin of the right coronary artery from the left sinus. Surgical correction of the anomaly was not required before replacement of the valve or proximal aorta. None of the individuals had a patent ductus arteriosus. One individual with bicuspid aortic valve type 1 (0.5 % of all individuals) had a previous surgery due to atrial septal defect at the age of 7 years. One other individual, also with type 1 bicuspid aortic valve (0.5 % of all individuals) had a ventricular septal defect, corrected at the age of 5 years. Mitral valve prolapse was identified in three (1.5%) individuals. All of these individuals had a bicuspid aortic valve type 1. Two individuals had a mild prolapse of the anterior leaflet without a relevant insufficiency and one individual had a prolapse of the posterior leaflet. This individual underwent surgical correction of the valve at the age of 35 years because of high grade insufficiency. There was no individual with tricuspid valve prolapse in our study collective. We did not observe a correlation between the bicuspid aortic valve type and occurrence of malformations.
Table 2
| Variable | No. of Individuals with findings (N=200*) |
|---|---|
| Aortic coarctation | 7/166 (4.2%) |
| Coronary artery anomaly | 3/84 (3.6%) |
| Patent ductus arteriosus | 0/164 |
| Atrial septal defect | 1 (0.5%) |
| Ventricular septal defect | 1 (0.5%) |
| Mitral valve prolapse | 3 (1.5%) |
| Tricuspid valve prolapse | 0 |
*, if less than total, we present the number of individuals with available information behind a slash.
Bicuspid aortic valve—associate malformations according to the literature
Our search of the literature yielded a total of 2,362 results (Figure 1). After exclusion of case reports, editorials, and reviews, we reviewed all 1,371 articles by title and abstract. We excluded 217 articles without abstract or full text available and further 1,085 with no concomitant malformations reported. From the remaining 69 full text articles we excluded 43 reports with small cohorts or with collectives that included predominantly individuals with syndromic diseases or complex malformation.
The systematic review includes a total of 26 studies that qualified for inclusion in the meta-analysis. We rated the selection bias for the study collectives as predominantly low- to intermediate (Table S1). The age of study individuals and primary imaging modalities are presented in the Table 3.
Table 3
| First author, year of publication | Age (years) | Males (%) | Primary imaging modality | |
|---|---|---|---|---|
| Median (range) | Mean ± SD | |||
| Coarctation of the aorta | ||||
| Roberts 1970 (37) | 46 (15 to 79) | n.a. | 72 | Necropsy |
| Pachulski 1993 (38) | 36 (21 to 67) | n.a. | 78 | TTE |
| Nistri 2005 (9) | n.a. | 18±1 | 100 | TTE |
| Ciotti 2006 (39) | 5 (0 to 37) | n.a. | 70 | TTE |
| Tzemos 2008 (40) | n.a. | 35±16 | 68 | TTE |
| Thanassoulis 2008 (41) | n.a. | 33±14 | 72 | TTE |
| Schaefer 2008 (21) | n.a. | BAV 1: 46±14; BAV 2: 43±15 | 70 | TTE |
| Michelena 2008 (42) | n.a. | 32±20 | 65 | TTE |
| Oliver 2009 (43) | 32 (18 to 51) | n.a. | 68 | TTE |
| Yuan 2010 (44) | n.a. (16 to 85) | 56±15 | 77 | TTE |
| Michelena 2011(45) | n. a. | 35±21 | 69 | TTE |
| Roberts 2012 (46) | n.a. (23 to 89) | 55±15 | 77 | Necropsy |
| Lee 2013 (1) | n.a. | 56±9 | 92 | TTE |
| Koenraadt 2016 (47) | n.a. (18 to 85) | 48±15 | 70 | TTE |
| Koenraadt 2016 (48) | n.a. | 51±14 | 79 | TTE |
| Masri 2016 (49) | n.a. | 50±14 | 75 | TTE |
| Niaz 2017 (50) | 12 (0 to 22) | n.a. | 67 | TTE |
| Tripathi 2018 (51) | 5 (0 to 17) | n.a. | 61 | TTE |
| Ram 2018 (52) | n.a. | 42±14 | 94 | TTE |
| Koenraadt 2019 (53) | n.a. | 42±15 | 76 | CT scan |
| Own data | 45 (14 to 80) | 45±16 | 77 | TTE |
| Coronary anomalies | ||||
| Roberts 2012 (46) | n.a. (23 to 89) | 55±15 | 77 | Necropsy |
| Naito 2018 (54) | 61±13 | (1 to 85) | 72 | TTE |
| Michałowska 2016 (55) | n.a. | 58±14 | n.a. | CT and CT-angiography |
| Own data | 45 (14 to 80) | 45±16 | 77 | TTE |
| Patent ductus arteriosus | ||||
| Roberts 1970 (37) | 46 (15 to 79) | n.a. | 72 | Necropsy |
| Ciotti 2006 (39) | 5 (0 to 37) | n.a. | 70 | TTE, paediatric |
| Niaz 2017 (50) | 12 (0 to 22) | n.a. | 67 | TTE |
| Ram 2018 (52) | n.a. | 42±14 | 94 | TTE |
| Own data | 45 (14 to 80) | 45±16 | 77 | TTE |
| Atrial septal defect | ||||
| Niaz 2017 (50) | 12 (0 to 22) | n.a. | 67 | TTE |
| Own data | 45 (14 to 80) | 45±16 | 77 | TTE |
| Ventricular septal defect | ||||
| Roberts 1970 (37) | 46 (15 to 79) | n.a. | 72 | Necropsy |
| Pachulski 1993 (38) | 36 (21 to 67) | n.a. | 78 | TTE |
| Lamas 2000 (56) | n.a. | 39±9 | 100 | TTE |
| Nistri 2005 (9) | n.a. | 18±1 | 100 | TTE |
| Ciotti 2006 (39) | 5 (0 to 37) | n. a. | 70 | TTE |
| Lee 2013 (1) | n.a. | 56±9 | 92 | TTE |
| Niaz 2017 (50) | 12 (0 to 22) | n.a. | 67 | TTE |
| Tripathi 2018 (51) | 4.7 (0 to 17) | n.a. | 61 | TTE |
| Own data | 45 (14 to 80) | 45±16 | 77 | TTE |
| Mitral valve prolapse | ||||
| Roberts 1970 (37) | 46 (15 to 79) | n.a. | 72 | Necropsy |
| Lamas 2000 (56) | n.a. | 39±9 | 100 | TTE |
| Lad 2009 (57)* | (21 to 74) | 51±15 | 86 | TTE |
| Van Rensburg 2017 (58) | 44 (1 to 9) | n.a. | 60 | TTE |
| Padang 2018 (59) | n.a. | 51±16 | 81 | TTE |
| Own data | 45 (14 to 80) | 45±16 | 77 | TTE |
*, the age range and percentage of males refers to the 29 individuals with MVP. Three individuals with MVP had a Marfan Syndrome. SD, standard deviation; n.a., not available; TTE, transthoracic chocardiography; BAV, bicuspid aortic valve; CT, computed tomography; MVP, mitral valve prolapse.
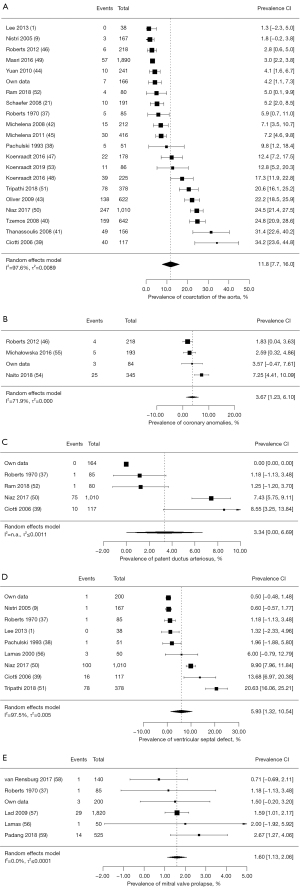
Our meta-analysis documented a pooled prevalence of 11.8% for aortic coarctation (95% CI: 7.7–16.0%), 3.67% for coronary anomalies (95% CI: 1.23–6.10%), 3.34% for patent ductus arteriosus (95% CI: 0.0–6.69%), 5.93% for ventricular septal defect (95% CI: 1.32–10.54%), and 1.6% for mitral valve prolapse (95% CI: 1.13–2.06%) (Figure 2). We found only one study which showed a prevalence of atrial septal defect with 7.5% (95% CI: 5.83–9.22%) in individuals with bicuspid aortic valve (Table 3). Therefore, a pooled prevalence could not be assessed.
Competing risks of surgeries for associated malformations versus bicuspid aortic valve disease in our retrospective study collective
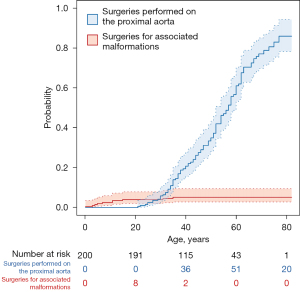
Surgeries for associated malformations were performed at a significantly younger age 12.2±11.8 years (range, 0.3–35 years) than those required for the bicuspid aortic valve disease, 48.0±13.2 years (range, 21–77 years), P<0.001.
The risk for surgeries for associated malformations increases before adolescence, whereas the risk for surgery for aortic valve or proximal aortic pathology starts to increase after 20 years of age (Figure 3).
Discussion
Our study reports results from comprehensive imaging in adolescents and adults with a bicuspid aortic valve that was considered as isolated cardiovascular malformation. Our investigation documents that bicuspidality of the aortic valve may indeed be associated with concomitant cardiovascular malformations. The meta-analysis of the literature supports these findings. Pooling of our data and those from the literature document aortic coarctation in 11.8%, coronary anomalies in 3.7%, patent ductus arteriosus in 3.3%, ventricular septal defect in 5.9%, atrial septal defect in 4.0%, and mitral valve prolapse in 1.6% of individuals with a bicuspid aortic valve.
Aortic coarctation has a worldwide reported prevalence of 0.034% (3.4/10,000 live births) (60). We found a noticeably higher rate of coarctation in bicuspid aortic valve disease. Our meta-analysis revealed considerable variability in the reported prevalence of aortic coarctation in bicuspid aortic valve disease. We explain these highly variable frequencies with heterogeneous study groups. For example, the cohort with the highest reported rate of 34.2% (39) included mostly children, 4.2% with a syndromic disease. In contrast, two other studies reported a prevalence ranging between 20% and 25% for aortic coarctation in bicuspid aortic valve, but did not provide information on the frequency of underlying syndromic conditions (50,51). Interestingly, even in the three studies, which strictly excluded syndromic anomalies, the rate of concomitant aortic coarctation was high with 31.4% (41), 24.8% (40), and 22.2% (43) in adults with bicuspid aortic valve disease. In line with our findings, these studies suggest an association of aortic coarctation and non-syndromic bicuspid aortic valve disease.
Coronary anomalies occur with a prevalence of 0.2–2.3% in the general population (61). The highest prevalence was reported with usage of magnetic resonance angiography, most likely because other imaging modalities may be less sensitive (62). With about 3.6% of individuals with bicuspid aortic valve in our study group and 3.7% in the meta-analysis exhibiting an anomaly of the coronary anatomy, we noted a slightly higher prevalence than in the general population. Indeed, a direct comparison of groups with bicuspid versus tricuspid aortic valves revealed a more frequent occurrence of coronary anomalies in the bicuspid valve group (7.2% in bicuspid vs. 2.8% in tricuspid aortic valve group) (54).
A persistent ductus arteriosus is diagnosed in 0.009% (0.87/10,000 live births) worldwide (60). Although none of the individuals in our study group had a persistent ductus arteriosus, our meta-analysis identified a rate of 3.3% in bicuspid aortic valve disease. However, the number of children was high in studies that support a high prevalence of persistent ductus arteriosus (39,50), whereas collectives with age ranges similar to ours showed a prevalence of 1.3% (37,52).
Atrial septal defect is diagnosed with a 0.016% prevalence (1.64/10,000 live births) worldwide (60). We identified only one individual (0.5%) with this anomaly. Our review of the literature included one study that assessed the occurrence of atrial septal defect in a group of predominantly children with bicuspid aortic valve disease. Here, the prevalence of atrial septal defect was 7.4%. However, complex cardiovascular anomalies were included in this study group.
Ventricular septal defect has a prevalence of 0.026% (2.62/10,000 live births) (60). Our study identified 0.5% of individuals with concomitant ventricular septal defect. Our meta-analysis revealed a pooled prevalence of 5.9%, where cohorts with more children exhibited a higher frequency of this congenital defect than those with more adults (3.8–20.6% vs. 0–6%) (39,51).
The prevalence of mitral valve prolapse in the general population is about 2.4% (84/3,491) and is more common in women (63). It ranges from 0.4% (5/1,382) among male to 1.3% (9/690) among female teenagers (64) and shows an up to 2.1% (34/1,646) prevalence in male and 2.7% (50/1,845) in female adults (32). Mitral valve prolapse was present in 1.5% of individuals in our study group, predominantly comprising male individuals with bicuspid aortic valves, and in 1.6% of individuals according to our meta-analysis. Thus, we found that mitral valve prolapse occurred with similar frequencies in bicuspid aortic valve disease and in the general population. Indeed, another study showed a comparable frequency of mitral valve prolapse in bicuspid (2.7%) and tricuspid (3.4%) aortic valves (63). Padang et al. describe elongated and at least slightly prolapsed anterior mitral valve leaflets and thereby suggest a specific mitral valve phenotype in bicuspid aortic valve disease rather than a more frequent occurrence of a prolapse (63).
Tricuspid valve prolapse is a far less common valve anomaly. Ong et al. (65) found a prevalence of 0.03% (20/63,472) in adults. Interestingly, tricuspid valve prolapse exclusively occurred in those individuals with a concomitant mitral valve prolapse. Given this small prevalence in the general population, we did not identify any individual with this anomaly in our study group and study data on the prevalence of tricuspid valve prolapse in bicuspid aortic valve disease are missing in the literature.
Competing risk of surgeries for associated malformations is the highest in childhood and decreases with age. These congenital defects tend to be symptomatic and thus require correction at a younger age. In contrast, the risk of surgery due to a pathology of the bicuspid aortic valve itself or a concomitant bicuspid aortopathy seems to increase with age. A possible explanation might be a progressive valve degeneration and dilatation of the aorta with increasing age.
Study limitations
Given the retrospective design of our study, all presented data have to be considered with caution. In many cases, we relied on patient charts regarding associated malformations diagnosed in childhood. Therefore, asymptomatic ventricular and septal defects with spontaneous closure may not have been considered. As we did not routinely perform bubble tests, we may also have missed some asymptomatic atrial septal defects. Only individuals with clinical indication underwent coronary artery imaging. This might have led to underestimation of asymptomatic coronary anomalies especially in young individuals with no risk factors for coronary artery disease.
We did not detect all the cardiovascular malformations listed as associates of bicuspid aortic valve disease, which may be explained by our comparatively small study group. Furthermore, we did not assess the frequency of aneurysms of brain vessels, another known malformation associated with bicuspid aortic disease (66).
In general, we found lower prevalences for malformations associated with bicuspid aortic valve disease in our own study collective than in our meta-analysis. We exclusively included individuals aged at least 14 years, whereas the meta-analysis also included neonates and children. If an associated malformation is diagnosed early, the individual tends to be followed up in an adult congenital heart center rather than in our cardiac outpatient department. Since some cohorts included in the meta-analysis are from specialized centers, a selection bias cannot be excluded. The overall prevalence in the general population might be slightly lower.
To gain a better insight into the prevalence of associated malformations in bicuspid aortic disease, population-based multicenter studies with a prospective design are needed.
Conclusions
In conclusion, individuals with isolated bicuspid aortic valve may exhibit a variety of associated cardiovascular malformations. In order to prevent later cardiac or intraoperative complications, screening for associated malformations may be warranted.
Acknowledgments
The authors thank Dr. Maria Wendt, Medical Writer, for writing assistance, language editing and proofreading.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Cardiovascular Diagnosis and Therapy for the series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part V”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE and PRISMA reporting checklists. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-112/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-112/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-112/coif). The series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part V” was commissioned by the editorial office without any funding or sponsorship. YVK served as the unpaid Guest Editor of this series. BT receives a project-related research grant by the German Heart Foundation outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee M, Sung J, Cho SJ, et al. Aortic dilatation and calcification in asymptomatic patients with bicuspid aortic valve: analysis in a Korean health screening population. Int J Cardiovasc Imaging 2013;29:553-60. [Crossref] [PubMed]
- Movahed MR, Hepner AD, Ahmadi-Kashani M. Echocardiographic prevalence of bicuspid aortic valve in the population. Heart Lung Circ 2006;15:297-9. [Crossref] [PubMed]
- Mordi I, Tzemos N. Bicuspid aortic valve disease: a comprehensive review. Cardiol Res Pract 2012;2012:196037. [Crossref] [PubMed]
- Kong WK, Regeer MV, Ng AC, et al. Sex Differences in Phenotypes of Bicuspid Aortic Valve and Aortopathy: Insights From a Large Multicenter, International Registry. Circ Cardiovasc Imaging 2017;10:e005155. [Crossref] [PubMed]
- Ramaraj R, Sorrell VL. Degenerative aortic stenosis. BMJ 2008;336:550-5. [Crossref] [PubMed]
- Sarajlic P, Wolk A, Bäck M, et al. Physical Activity Does Not Reduce Aortic Valve Stenosis Incidence. Circ J 2018;82:2372-4. [Crossref] [PubMed]
- Bravo-Jaimes K, Prakash SK. Genetics in bicuspid aortic valve disease: Where are we? Prog Cardiovasc Dis 2020;63:398-406. [Crossref] [PubMed]
- Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med 2014;370:1920-9. [Crossref] [PubMed]
- Nistri S, Basso C, Marzari C, et al. Frequency of bicuspid aortic valve in young male conscripts by echocardiogram. Am J Cardiol 2005;96:718-21. [Crossref] [PubMed]
- Cozijnsen L, van der Zaag-Loonen HJ, Braam RL, et al. Yield of family screening in patients with isolated bicuspid aortic valve in a general hospital. Int J Cardiol 2018;255:55-8. [Crossref] [PubMed]
- Zhang X, Zhu M, He T, et al. Cardiac Mechanics in Isolated Bicuspid Aortic Valve Disease With Normal Ejection Fraction: A Study of Various Valvular Lesion Types. Medicine (Baltimore) 2015;94:e2085. [Crossref] [PubMed]
- Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol 2010;55:2789-800. [Crossref] [PubMed]
- Roos-Hesselink JW, Schölzel BE, Heijdra RJ, et al. Aortic valve and aortic arch pathology after coarctation repair. Heart 2003;89:1074-7. [Crossref] [PubMed]
- Topaz O, DeMarchena EJ, Perin E, et al. Anomalous coronary arteries: angiographic findings in 80 patients. Int J Cardiol 1992;34:129-38. [Crossref] [PubMed]
- Duran AC, Frescura C, Sans-Coma V, et al. Bicuspid aortic valves in hearts with other congenital heart disease. J Heart Valve Dis 1995;4:581-90. [PubMed]
- Neumayer U, Stone S, Somerville J. Small ventricular septal defects in adults. Eur Heart J 1998;19:1573-82. [Crossref] [PubMed]
- Eichhorn P, Vogt P, Ritter M, et al. Abnormalities of the atrial septum in adults: kind, prevalence and clinical relevance. Schweiz Med Wochenschr 1995;125:1336-41. [PubMed]
- Chisholm JC. Mitral valve prolapse syndrome associated with congenital bicuspid aortic valve. J Natl Med Assoc 1981;73:921-3. [PubMed]
- Boudoulas KD, Borer JS, Boudoulas H. Etiology of valvular heart disease in the 21st century. Cardiology 2013;126:139-52. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495-9. [Crossref] [PubMed]
- Schaefer BM, Lewin MB, Stout KK, et al. The bicuspid aortic valve: an integrated phenotypic classification of leaflet morphology and aortic root shape. Heart 2008;94:1634-8. [Crossref] [PubMed]
- Lancellotti P, Tribouilloy C, Hagendorff A, et al. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2013;14:611-44. [Crossref] [PubMed]
- Ross DN. Replacement of aortic and mitral valves with a pulmonary autograft. Lancet 1967;2:956-8. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. [Crossref] [PubMed]
- Rodríguez-Palomares JF, Teixidó-Tura G, Galuppo V, et al. Multimodality Assessment of Ascending Aortic Diameters: Comparison of Different Measurement Methods. J Am Soc Echocardiogr 2016;29:819-826.e4. [Crossref] [PubMed]
- Goldstein SA, Evangelista A, Abbara S, et al. Multimodality imaging of diseases of the thoracic aorta in adults: from the American Society of Echocardiography and the European Association of Cardiovascular Imaging: endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2015;28:119-82. [Crossref] [PubMed]
- Kühne K, Keyser B, Groene EF, et al. FBN1 gene mutation characteristics and clinical features for the prediction of mitral valve disease progression. Int J Cardiol 2013;168:953-9. [Crossref] [PubMed]
- Weinrich JM, Lenz A, Girdauskas E, et al. Current and Emerging Imaging Techniques in Patients with Genetic Aortic Syndromes. Rofo 2020;192:50-8. [Crossref] [PubMed]
- Gach P, Dabadie A, Sorensen C, et al. Multimodality imaging of aortic coarctation: From the fetus to the adolescent. Diagn Interv Imaging 2016;97:581-90. [Crossref] [PubMed]
- Angelini P. Coronary artery anomalies: an entity in search of an identity. Circulation 2007;115:1296-305. [Crossref] [PubMed]
- Lancellotti P, Moura L, Pierard LA, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 2010;11:307-32. [Crossref] [PubMed]
- Freed LA, Levy D, Levine RA, et al. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med 1999;341:1-7. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- RCT. R Foundation for Statistical Computing. Vienna, Austria, 2021. Available online: https://www.r-project.org/
- Therneau TM, Lumley T, Atkinson E, Crowson, et al. A Package for Survival Analysis in R. R package version 3.2-13. Available online: https//CRANR-projectorg/package=survival
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software 2010;36:1-48. 1. Available online: https://www.jstatsoft.org/v36/i03/
- Roberts WC. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol 1970;26:72-83. [Crossref] [PubMed]
- Pachulski RT, Chan KL. Progression of aortic valve dysfunction in 51 adult patients with congenital bicuspid aortic valve: assessment and follow up by Doppler echocardiography. Br Heart J 1993;69:237-40. [Crossref] [PubMed]
- Ciotti GR, Vlahos AP, Silverman NH. Morphology and function of the bicuspid aortic valve with and without coarctation of the aorta in the young. Am J Cardiol 2006;98:1096-102. [Crossref] [PubMed]
- Tzemos N, Therrien J, Yip J, et al. Outcomes in adults with bicuspid aortic valves. JAMA 2008;300:1317-25. [Crossref] [PubMed]
- Thanassoulis G, Yip JW, Filion K, et al. Retrospective study to identify predictors of the presence and rapid progression of aortic dilatation in patients with bicuspid aortic valves. Nat Clin Pract Cardiovasc Med 2008;5:821-8. [Crossref] [PubMed]
- Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008;117:2776-84. [Crossref] [PubMed]
- Oliver JM, Alonso-Gonzalez R, Gonzalez AE, et al. Risk of aortic root or ascending aorta complications in patients with bicuspid aortic valve with and without coarctation of the aorta. Am J Cardiol 2009;104:1001-6. [Crossref] [PubMed]
- Yuan SM, Jing H, Lavee J. The bicuspid aortic valve and its relation to aortic dilation. Clinics (Sao Paulo) 2010;65:497-505. [Crossref] [PubMed]
- Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011;306:1104-12. [Crossref] [PubMed]
- Roberts WC, Vowels TJ, Ko JM. Natural history of adults with congenitally malformed aortic valves (unicuspid or bicuspid). Medicine (Baltimore) 2012;91:287-308. [Crossref] [PubMed]
- Koenraadt WM, Grewal N, Gaidoukevitch OY, et al. The extent of the raphe in bicuspid aortic valves is associated with aortic regurgitation and aortic root dilatation. Neth Heart J 2016;24:127-33. [Crossref] [PubMed]
- Koenraadt WM, Tokmaji G, DeRuiter MC, et al. Coronary anatomy as related to bicuspid aortic valve morphology. Heart 2016;102:943-9. [Crossref] [PubMed]
- Masri A, Kalahasti V, Alkharabsheh S, et al. Characteristics and long-term outcomes of contemporary patients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2016;151:1650-1659.e1. [Crossref] [PubMed]
- Niaz T, Poterucha JT, Johnson JN, et al. Incidence, morphology, and progression of bicuspid aortic valve in pediatric and young adult subjects with coexisting congenital heart defects. Congenit Heart Dis 2017;12:261-9. [Crossref] [PubMed]
- Tripathi A, Wang Y, Jerrell JM. Population-based treated prevalence, risk factors, and outcomes of bicuspid aortic valve in a pediatric Medicaid cohort. Ann Pediatr Cardiol 2018;11:119-24. [Crossref] [PubMed]
- Ram E, Sternik L, Lipey A, et al. Clinical and Echocardiographic Outcomes after Aortic Valve Repair in Patients with Bicuspid or Unicuspid Aortic Valve. Isr Med Assoc J 2018;20:423-8. [PubMed]
- Koenraadt WMC, Siebelink HJ, Bartelings MM, et al. Coronary anatomy in Turner syndrome versus patients with isolated bicuspid aortic valves. Heart 2019;105:701-7. [Crossref] [PubMed]
- Naito S, Petersen J, Reichenspurner H, et al. The impact of coronary anomalies on the outcome in aortic valve surgery: comparison of bicuspid aortic valve versus tricuspid aortic valve morphotype. Interact Cardiovasc Thorac Surg 2018;26:617-22. [Crossref] [PubMed]
- Michałowska IM, Hryniewiecki T, Kwiatek P, et al. Coronary Artery Variants and Anomalies in Patients With Bicuspid Aortic Valve. J Thorac Imaging 2016;31:156-62. [Crossref] [PubMed]
- Lamas CC, Eykyn SJ. Bicuspid aortic valve--A silent danger: analysis of 50 cases of infective endocarditis. Clin Infect Dis 2000;30:336-41. [Crossref] [PubMed]
- Lad V, David TE, Vegas A. Mitral regurgitation due to myxomatous degeneration combined with bicuspid aortic valve disease is often due to prolapse of the anterior leaflet of the mitral valve. Ann Thorac Surg 2009;87:79-82. [Crossref] [PubMed]
- van Rensburg A, Herbst P, Doubell A. A retrospective analysis of mitral valve pathology in the setting of bicuspid aortic valves. Echo Res Pract 2017;4:21-8. [Crossref] [PubMed]
- Padang R, Gersh BJ, Ommen SR, et al. Prevalence and Impact of Coexistent Bicuspid Aortic Valve in Hypertrophic Cardiomyopathy. Heart Lung Circ 2018;27:33-40. [Crossref] [PubMed]
- van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol 2011;58:2241-7. [Crossref] [PubMed]
- Yildiz A, Okcun B, Peker T, et al. Prevalence of coronary artery anomalies in 12,457 adult patients who underwent coronary angiography. Clin Cardiol 2010;33:E60-4. [Crossref] [PubMed]
- Graidis C, Dimitriadis D, Karasavvidis V, et al. Prevalence and characteristics of coronary artery anomalies in an adult population undergoing multidetector-row computed tomography for the evaluation of coronary artery disease. BMC Cardiovasc Disord 2015;15:112. [Crossref] [PubMed]
- Padang R, Enriquez-Sarano M, Pislaru SV, et al. Coexistent bicuspid aortic valve and mitral valve prolapse: epidemiology, phenotypic spectrum, and clinical implications. Eur Heart J Cardiovasc Imaging 2019;20:677-86. [Crossref] [PubMed]
- Sattur S, Bates S, Movahed MR. Prevalence of mitral valve prolapse and associated valvular regurgitations in healthy teenagers undergoing screening echocardiography. Exp Clin Cardiol 2010;15:e13-5. [PubMed]
- Ong K, Yu G, Jue J. Prevalence and spectrum of conditions associated with severe tricuspid regurgitation. Echocardiography 2014;31:558-62. [Crossref] [PubMed]
- Egbe AC, Padang R, Brown RD, et al. Prevalence and predictors of intracranial aneurysms in patients with bicuspid aortic valve. Heart 2017;103:1508-14. [Crossref] [PubMed]


