Catheter directed interventions for inferior vena cava thrombosis
Introduction
Inferior vena cava (IVC) thrombosis is an underdiagnosed condition associated with a mortality rate approaching twice that of lower extremity deep venous thrombosis (DVT) (1). The lack of a standard diagnostic approach, a sparsity of data, and nonspecific clinical presentation have all hindered in its clinical recognition. The incidence of IVC thrombosis in patients with confirmed DVT is estimated to be between 4–15% (2,3), which is likely an underestimate due to the lack of standard diagnostic protocols for caval thrombus.
Etiology
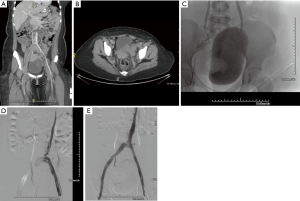
Thrombosis of the IVC can affect 60–80% of patients with congenital anomalies, especially in cases of IVC hypoplasia or aplasia (4). These anomalies can remain subclinical for many years due to well-developed collaterals. However, despite these collaterals, blood flow from the lower extremities eventually becomes inadequate, resulting in venous hypertension, stasis, and subsequent thrombosis (Figure 1). Congenital anomalies of the IVC are rare and can be associated with other congenital anomalies such as situs inversus, congenital heart diseases, polysplenia and asplenia (5,6).
Thrombotic occlusion related to indwelling IVC filters is the most common cause of acquired IVC thrombosis (7). When anticoagulation is contraindicated or ineffective in patients with or at high risk for venous thromboembolism, an IVC filter device may be considered. In the past, this was performed via surgical ligation of the IVC, which was associated with a high rate of mortality as well as chronic venous insufficiency. In the 1960’s, partial interruption of the IVC was introduced using suture and caval clips, though associated IVC occlusion rates remained unacceptably high. The Mobin-Uddin umbrella device was developed in 1967, which partially interrupted flow in the IVC but was also associated with a high percentage of caval thrombus due to inadequate flow. The Greenfield filter was introduced in 1971, followed by the percutaneous Greenfield filter in 1981, which allowed for more blood flow while filtering and lowering the rate of caval thromboses (8).
Thrombotic complications remain the most common adverse event related to IVC filter placement. IVC occlusions occur in up to 2.7% of cases in 6–24 months after placement (9). The increased incidence of deep-vein thrombosis in patients with vena cava filters may be related to the thrombotic occlusion of those filters leading to venous stasis in the legs. In the PREPIC study, a randomized controlled trial examining efficacy of IVC filters, 26 of 57 patients with deep-vein thrombosis in the IVC filter arm experienced filter thrombosis (10). Despite the increased incidence of thrombus in patients with IVC filters, the presence of a filter alone is not considered an indication for long-term anticoagulation (11). Results of the PREPIC study suggested that an IVC filter is beneficial during the early course of an acute DVT in patients that can’t be anticoagulated. Later on the filter is more likely to cause thrombosis of the cava than to prevent a pulmonary embolus (PE). These data have led to the increased use of optional filters that can be removed when the filter is no longer deemed necessary. Currently, approximately 50% or less of retrievable filters placed are ever removed (12). It is unclear what the true risk of thrombus over time is, however, as with any chronic implant, the risk would be expected to increase with length of implantation. In 2010 the FDA released a safety communication, with a follow-up in 2014, detailing possible complications of long-term indwelling IVC filters. This release encouraged all physicians involved in the treatment, and follow-up of these patients, to consider removing filters when it is no longer needed (13). Increased vigilance in retrieving unnecessary filters may decrease associated long-term complications. The PRESERVE study is a large scale multi-specialty prospective trial evaluating IVC filter use with long-term follow-up. This study is currently enrolling subjects in the United States and may provide further data about IVC filter complications (14).
Acquired IVC thrombosis can also result from cranial extension of DVT into the IVC, spontaneous thrombosis of the cava, venous stasis in the setting of external compression, or thrombosis secondary to a thrombogenic diseased or injured vessel wall (15). Spontaneous thrombosis is typically related to Virchow’s triad of hypercoagulability, stasis and endothelial injury. Pro-thrombotic factors include: thrombophilia, malignancy, oral contraceptives, smoking, obesity, pregnancy, hormonal replacement therapy and nephrotic syndrome. External compression may be a sequelae of renal cell tumors, uterine fibroids, hematoma, or compression from other vascular structures such as an abdominal aortic aneurysm or an iliac artery such as in May-Thurner syndrome (16).
Clinical presentation
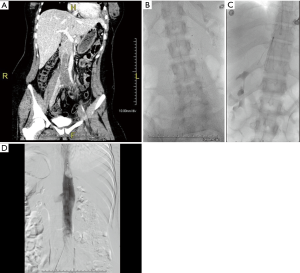
The clinical presentation of IVC thrombosis varies according to the anatomical level of thrombosis, the degree of occlusion and chronicity of the thrombus. Acute IVC thrombosis is generally accompanied by a sudden onset of symptoms, while chronic IVC thrombosis, presents with a more gradual onset, secondary to thrombotic IVC occlusion with already established venous collateralization or gradual narrowing of the IVC (15). As with DVT, the typical presentation of IVC thrombosis may present with pain, swelling, and cramping of the lower extremities. In addition, and more specific to IVC thrombosis, back and pelvic pain are generally the earliest symptoms. In cases that have renal vein involvement, symptoms may involve hematuria and flank pain whereas bilateral renal venous thrombosis can result in acute renal failure (17). Pulmonary embolism, which is reported in up to 12% of patients with IVC thrombosis, can present with chest pain and shortness of breath (18). Chronic occlusion can have varied presentations depending on the extent of the collateral venous drainage. Patients can present with bilateral lower extremity pain and edema; skin changes such as pigmentation and venous ulcers; back pain; inguinal, abdominal and thoracic varicosities. Rarely, chronic IVC thrombosis can present with compromised kidney or liver function (Budd-Chiari syndrome) if the venous outflow from these organs are obstructed (Figure 2). In addition, patients with chronic thrombosis can present with severe lumbar radicular pain, sciatica, or cauda-equina symptoms secondary to compression from paraspinal collaterals (19). With chronically untreated IVC thrombosis, patients are at risk of developing post-thrombotic syndrome (PTS) as a result of chronic venous hypertension and stasis. Presenting symptoms range from minor leg heaviness to debilitating leg claudication.

Diagnosis
Although no specific diagnostic guidelines for IVC thrombosis exist, non-invasive imaging modalities such as ultrasound (US), contrast enhanced computer tomography (CECT), and magnetic resonance venography (MRV) have resulted in higher and more accurate diagnostic rates of IVC thrombosis. Invasive venography is generally reserved for intent to treat or, in rare cases, a problem solver when other modalities are inconclusive.
Duplex US is often the first-line diagnostic imaging modality, providing a rapid, readily available, non-ionizing, and non-invasive method. US may also detect pathology causing thrombosis such as extrinsic compression of the IVC or anomalies of the IVC itself. However, it suffers from inter-user variability due to its operator-dependent nature. Accurate visualization of the IVC on US can also be limited by overlying bowel gas and patient body habitus (20).
CECT has excellent contrast resolution, allowing visualization of venous structures as well as the ability to depict secondary abdominal or pelvic pathology (21). CECT also has the ability to differentiate between bland and tumor thrombus by observing for enhancement during the arterial phase of injection. CECT is also advantageous in patients with venous stents compared with MRI which may be prone to susceptibility artifact in these areas. CECT is prone to over diagnosing IVC thrombosis, due to the merging of contrast enhanced blood with unenhanced venous return from the lower body (22). Longer delays in imaging after contrast injection make opacification more homogeneous. Peak opacification time ranges from 93–147 seconds while scanning at 210 seconds results in more homogenous opacification which is most likely optimal (23).
MRV evaluation of the IVC can be performed with or without gadolinium enhanced sequences. MRV is advantageous as it does not use ionizing radiation, provides more accurate delineation of thrombus, as well as accurate visualization of IVC anomalies. It can be used to follow morphological changes of thrombus after therapy as well (24). In patients with an existing IVC filter or venous stent, susceptibility artifact may limit accurate assessment of adjacent veins. In addition, availability and cost are other limitations to routinely using MRV.
Treatment
If left untreated, most patients with IVC thrombus develop some sequelae of PTS (25). The cornerstone of treatment centers on systemic anticoagulation therapy to reduce thrombus propagation and minimize the risk of pulmonary embolization while alleviating lower extremity symptoms. The open vein hypothesis of DVT (26) would seem to translate to patients with IVC thrombosis as well, in that long term outcomes would improve with more aggressive endovascular treatment involving thrombolysis or thrombectomy.
Patients with IVC thrombosis have a large thrombus burden and thus are at significant risk of thrombosis-related morbidity and mortality. In addition, they often have a correctable risk factor for rethrombosis. Simultaneously addressing both these factors, results in rapid symptom relief with improved patency and lower incidence of PTS (27).
In chronic IVC thrombus, the removal of the occlusion allows for less impeded venous flow, thereby decreasing venous hypertension and improving associated symptoms.
Acute thrombosis
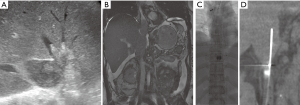
Endovascular techniques in treating acute IVC thrombus are similar to those described in treating acute iliofemoral DVT, though significant differences exist due to the larger clot burden in the cava. Caval thrombi carry a greater risk of lethal pulmonary embolism and can also involve the renal veins. Although placement of IVC filter for endovascular treatment of iliofemoral vein thrombosis may not be indicated (28), consideration for placing a filter should be made when treating IVC thrombus given the more extensive and possibly free floating clot (Figure 3). In addition, catheter directed lytic therapies in the IVC may require longer lysis times because of the large thrombus burden and mechanical thrombectomy devices would need to have a larger radial reach to treat pockets of thrombus in the larger caliber vessel.
Catheter directed thrombolysis (CDT) delivers thrombolytics directly into the thrombus, thereby providing a safer alternative to systemic thrombolysis. A multiple sidehole infusion catheter is positioned within the thrombus and thrombolytic agent is infused directly into the thrombus. This is routinely performed from a femoral or popliteal vein approach, depending on the extent of the clot. Venography can be repeated at 12–24 hours, and if there is significant residual clot, CDT may be continued. However, lengthy thrombolysis increases the rate of bleeding complications (16).
US assisted thrombolysis (EKOS) (BTG, UK) entails technology in which high frequency US disrupts the fibrin matrix of the thrombus, thereby exposing more binding sites for the thrombolytics (29,30). Compared with CDT, EKOS displays similar efficacy and potentially shorter treatment duration (31) which may be advantageous, specifically in the setting of large thrombus burden (32).
In light of the bleeding risks associated with prolonged thrombolytic infusions to treat extensive thrombus, percutaneous mechanical thrombectomy (PMT) devices can be used as an adjunct or alternative. The AngioJet rheolytic thrombectomy device (Boston Scientific, Marlborough, MA, USA) utilizes high-pressure pulsatile saline jets to create localized low-pressure zones for thrombus maceration and aspiration. Effectiveness of the device may be limited in large spaces such as the vena cava, in that the diameter of the action of thrombectomy does not reach the entire diameter of the cava, and will often create a channel through the clot. A spiral technique can be utilized in which the AngioJet is placed within a curved sheath to direct it towards pockets of thrombi as the catheter is withdrawn. With large IVC clots, care must be taken in the amount of time thrombectomy is performed with AngioJet, as the rheolytic mechanism causes hemolysis. This may precipitate acute renal failure (33) in patients at risk, as well as a drop in hemoglobin levels. The device also allows for a power pulse spray feature in which thrombolytics can be infused with a high pressure spray directly with deep penetration of the lytic agent into the thrombus. After allowing the thrombus to bathe in thrombolytics, the catheter can then be switched to the thrombectomy setting to reduce residual clot. Combining power-pulse with thrombectomy can reduce the amount of hemolysis (34). A recently introduced more powerful 8 F AngioJet catheter named the ZelanteDVT is likely to be more effective in large spaces such as the IVC, although experience is currently limited.
The AngioVac system (AngioDynamics, Latham, New York, USA) consists of a 22 F cannula with an expandable tip that opens to 48 F and creates a suction vortex allowing en-bloc removal of a large amount of thrombus while maintaining flow during extracorporeal circulation. By using the AngioVac to aspirate caval thrombus, the use of thrombolytics can be avoided in patients who may be at risk of bleeding while also avoiding the hemolysis associated with rheolytic devices. The suction vortex can pull in the walls of the vein to be able to reach the clot in a large vessel such as the IVC (35). Limitations include the large size and rigidity of the cannula precluding passage of the device into leg veins, thereby necessitating other techniques or devices to treat the inflow. In addition, these procedures must be performed with perfusionists as well as under general anesthesia.
Other thrombectomy devices successfully used to treat caval thrombus utilize rotating sinusoidal wires to macerate the clot (36,37). These devices can be used in conjunction with thrombolytic agents for better dispersal throughout the clot. The CleanerXT/Cleaner15 (Argon Medical Devices, Athens, TX, USA) is comprised of a single unit rotating S-wire and the drive motor with a side port which allows infusion of thrombolytics or contrast media. While effective at clearing thrombus, this device potentially can cause emboli and insertion of IVC filter should be considered when using this device to treat DVT (36). The Trellis device (Medtronic, Dublin, Ireland) also utilizes rotating sinusoidal wires but has proximal and distal occluding balloons which allow the infusion of thrombolytic agents to the isolated thrombosed vascular segment and protect clot from embolizing. However, in the large caliber IVC, the balloons may not always be capable of being occlusive. The device is currently off the market due to a class I FDA recall as of February 2015, due to mislabeling of balloon inflation ports. Both of these devices are also limited for treating clot within a thrombosed filter.
Chronic thrombosis
Acute soft thrombi begin to undergo fibrotic transformation within 2 weeks of clot formation. While catheter based thrombectomy can be very effective in the treatment of soft acute thrombus, the chronic fibrotic component remains more difficult to treat. Chronic DVT can cause luminal stenosis, stasis and predispose to recurrent DVT. The current treatment of choice for chronic, symptomatic iliocaval thrombosis is endovascular angioplasty combined with stenting.
Surgical reconstruction of central venous occlusion is associated with significant morbidity and marginal results. Gloviczki et al. reported limited patency of IVC reconstructions following surgical reconstruction in 28 patients with SVC or iliocaval obstruction (38). Long-term patency of angioplasty alone in the setting of chronic occlusion is limited due to venous recoil, low flow, and thrombogenicity of the lumen (39). Stents have the potential to improve patency in large veins by preventing recoil and allowing sufficient flow to maintain patency. The literature supports stenting and angioplasty in the IVC with improvement of symptoms and good patency (40). When chronic IVC obstruction cannot be recanalized via endovascular means, stenting to collateral drainage, possibly to the ascending lumbar vein, has been advocated with improvement in symptoms and good patency.
Choice devices for stenting the cava currently include WallStent (Boston Scientific Marlborough, MA, USA), and Gianturco-Rosch tracheobronchial Z stents (Cook, Bloomington, IN, USA) (41). Both have adequate radial force and are available in appropriate sizes. Z stents have the advantage of having large interstices that can be deployed across veins without compromising inflow. Z stents have a greater radial force than Wallstents and do not foreshorten upon deployment, making precise placement easier. The disadvantages of Z stents include large caliber sheaths, which are necessary for delivery, as well as the stabilizing barbs which although decrease the risk of migration, carry a theoretical predisposition to perforation.
Migration of the stent into the right heart or pulmonary arteries is one of the most dreaded complications. Thus, stenting the suprarenal IVC must be done cautiously, as there is a higher risk for stent migration in this region (42). Wallstents in particular may undergo unpredictable foreshortening upon expansion and may not adequately fix to the caval wall (43). Palmaz stents have been utilized to treat short segmental occlusions (44). However, they are not an ideal choice for lesions at the IVC/right atrial junction given reports of stent migration at this location (45). Deploying self expanding IVC stents from a right internal jugular vein approach, especially when the vessel is structured, may be advantageous as it allows the stent to first fix to the caudal part of the cava below the stricture. If however, it fixes to the cranial portion first, the stent may “watermelon seed” and migrate. In addition, if the stent jumps forward upon deployment, it will extend caudally, as opposed to towards the heart.
The data regarding placing stent grafts in the IVC is limited. Due to the nature of covered stents, the orifice of collateral veins which may have developed to compensate, may be covered, possibly exacerbating symptoms. In addition, if the grafts are oversized, any redundancy in the material which may not be fully expanded can serve as a nidus for thrombus in a low pressure venous system.
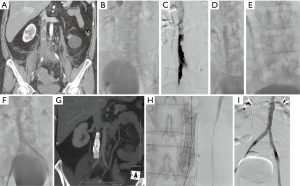
A specific technical problem is encountered in the setting of an occluded IVC filter. The obstruction needs to be relieved in order to assure adequate outflow. Angioplasty and stenting through the filter to restore flow has been shown to be safe with good patency both in patients with acute thrombosis after thrombolysis (46) and in patients with chronic IVC occlusion with indwelling IVC filter (47) (Figure 4).
Tumor thrombus
Tumors of the IVC are rare, with surgical resection generally the only alternative for cure or palliation of symptoms (48). Malignancies can be primary, such as leiomyosarcomas, or secondary, such as retroperitoneal soft tissue hepatic tumors or pancreaticoduodenal cancers. Other malignancies, such as renal cell carcinoma, pheochromocytoma, adrenocortical carcinoma, uterine sarcomas and germ cell tumors, also cause tumor thrombi.
The role for endovascular treatment is limited in certain cases, it may be difficult to discern between bland and tumor thrombus and the interventionalist may be consulted to evaluate for treatment. Demonstrating enhancement of thrombus on CT or MRI can help make the diagnosis of tumor thrombus. In addition, if histologic tissue diagnosis is helpful for treatment planning, a core needle biopsy can be obtained intravenously using a transjugular liver biopsy system (Figure 5). In instances where a mobile tumor thrombus protrudes into the right atrium and the risk for embolization or tricuspid valvular damage exists, AngioVac can be used for removal (49,50).
Conclusions
IVC thrombosis can be associated with significant morbidity if left untreated. As no standardized diagnostic guidelines exist, clinicians must maintain a high index of suspicion across a wide range of different clinical presentations to expeditiously and effectively treat this under-recognized condition. US, CECT, MRV, and invasive venography are all modalities at our disposal for further workup and characterization. Endovascular techniques can rapidly and safely remove IVC thrombi, thereby treating possible underlying venous occlusions, alleviating symptoms, and reducing the likelihood of complications with chronic thrombus such as PTS.
Acknowledgements
None.
Footnote
Conflicts of Interest: Y Golowa involved in a clinical trial with the AngioVac (Angiodynamics). The other authors have no conflicts of interest to declare.
References
- Agnelli G, Verso M, Ageno W, et al. The MASTER registry on venous thromboembolism: description of the study cohort. Thromb Res 2008;121:605-10. [Crossref] [PubMed]
- Anderson FA Jr, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med 1991;151:933-8. [Crossref] [PubMed]
- White RH. The epidemiology of venous thromboembolism. Circulation 2003;107:I4-8. [Crossref] [PubMed]
- Sitwala PS, Ladia VM, Brahmbhatt PB, et al. Inferior vena cava anomaly: a risk for deep vein thrombosis. N Am J Med Sci 2014;6:601-3. [Crossref] [PubMed]
- Chee YL, Culligan DJ, Watson HG. Inferior vena cava malformation as a risk factor for deep venous thrombosis in the young. Br J Haematol 2001;114:878-80. [Crossref] [PubMed]
- Gayer G, Luboshitz J, Hertz M, et al. Congenital anomalies of the inferior vena cava revealed on CT in patients with deep vein thrombosis. AJR Am J Roentgenol 2003;180:729-32. [Crossref] [PubMed]
- Stein PD, Matta F, Yaekoub AY. Incidence of vena cava thrombosis in the United States. Am J Cardiol 2008;102:927-9. [Crossref] [PubMed]
- Cimochowski GE, Evans RH, Zarins CK, et al. Greenfield filter versus Mobin-Uddin umbrella: the continuing quest for the ideal method of vena caval interruption. J Thorac Cardiovasc Surg 1980;79:358-65. [PubMed]
- Morales JP, Li X, Irony TZ, et al. Decision analysis of retrievable inferior vena cava filters in patients without pulmonary embolism. J Vasc Surg Venous Lymphat Disord 2013;1:376-84. [Crossref] [PubMed]
- PREPIC Study Group. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d'Embolie Pulmonaire par Interruption Cave) randomized study. Circulation 2005;112:416-22. [Crossref] [PubMed]
- Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:7S-47S.
- Kaufman JA, Rundback JH, Kee ST, et al. Development of a research agenda for inferior vena cava filters: proceedings from a multidisciplinary research consensus panel. J Vasc Interv Radiol 2009;20:697-707. [Crossref] [PubMed]
- U.S. Food and Drug Administration. Removing retrievable inferior vena cava filters: initial communication. 09 Aug. 2010. Accessed July 25, 2016. Available online: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm221676.htm.
- PRESERVE. Predicting the Safety and Effectiveness of Inferior Vena Cava Filters. Accessed July 25, 2016. Available online: http://www.preservetrial.com/
- Browse NL, Burnand KG, Irvine AT, et al. editors. Deep vein thrombosis: Pathology. Diseases of the Veins, 2nd edition. London: Arnold Publishers, 1999:249-89.
- Alkhouli M, Bashir R. Inferior vena cava filters in the United States: less is more. Int J Cardiol 2014;177:742-3. [Crossref] [PubMed]
- Marcy PY, Magné N, Frenay M, et al. Renal failure secondary to thrombotic complications of suprarenal inferior vena cava filter in cancer patients. Cardiovasc Intervent Radiol 2001;24:257-9. [Crossref] [PubMed]
- Chan WS, Ginsberg JS. Diagnosis of deep vein thrombosis and pulmonary embolism in pregnancy. Thromb Res 2002;107:85-91. [Crossref] [PubMed]
- Go MR, Baril DT, Leers SA, et al. Acute cauda equina syndrome secondary to iliocaval thrombosis successfully treated with thrombolysis and pharmacomechanical thrombectomy. J Endovasc Ther 2009;16:233-7. [Crossref] [PubMed]
- Park JH, Lee JB, Han MC, et al. Sonographic evaluation of inferior vena caval obstruction: correlative study with vena cavography. AJR Am J Roentgenol 1985;145:757-62. [Crossref] [PubMed]
- Giordano P, Weber K, Davis M, et al. Acute thrombosis of the inferior vena cava. Am J Emerg Med 2006;24:640-2. [Crossref] [PubMed]
- Kandpal H, Sharma R, Gamangatti S, et al. Imaging the inferior vena cava: a road less traveled. Radiographics 2008;28:669-89. [Crossref] [PubMed]
- Szapiro D, Ghaye B, Willems V, et al. Evaluation of CT time-density curves of lower-limb veins. Invest Radiol 2001;36:164-9. [Crossref] [PubMed]
- Soler R, Rodríguez E, López MF, et al. MR imaging in inferior vena cava thrombosis. Eur J Radiol 1995;19:101-7. [Crossref] [PubMed]
- Chuang VP, Mena CE, Hoskins PA. Congenital anomalies of the inferior vena cava. Review of embryogenesis and presentation of a simplified classification. Br J Radiol 1974;47:206-13. [Crossref] [PubMed]
- Sista AK, Vedantham S, Kaufman JA, et al. Endovascular Interventions for Acute and Chronic Lower Extremity Deep Venous Disease: State of the Art. Radiology 2015;276:31-53. [Crossref] [PubMed]
- Alkhouli M, Zack CJ, Zhao H, et al. Comparative outcomes of catheter-directed thrombolysis plus anticoagulation versus anticoagulation alone in the treatment of inferior vena caval thrombosis. Circ Cardiovasc Interv 2015;8:e001882. [Crossref] [PubMed]
- Protack CD, Bakken AM, Patel N, et al. Long-term outcomes of catheter directed thrombolysis for lower extremity deep venous thrombosis without prophylactic inferior vena cava filter placement. J Vasc Surg 2007;45:992-7; discussion 997. [Crossref] [PubMed]
- Lauer CG, Burge R, Tang DB, et al. Effect of ultrasound on tissue-type plasminogen activator-induced thrombolysis. Circulation 1992;86:1257-64. [Crossref] [PubMed]
- Suchkova V, Siddiqi FN, Carstensen EL, et al. Enhancement of fibrinolysis with 40-kHz ultrasound. Circulation 1998;98:1030-5. [Crossref] [PubMed]
- Parikh S, Motarjeme A, McNamara T, et al. Ultrasound-accelerated thrombolysis for the treatment of deep vein thrombosis: initial clinical experience. J Vasc Interv Radiol 2008;19:521-8. [Crossref] [PubMed]
- Latchana N, Dowell JD, Al Taani J, et al. Ultrasound-accelerated, catheter-directed thrombolysis for inferior vena cava thrombosis after an orthotopic liver transplant. Exp Clin Transplant 2015;13:96-9. [PubMed]
- Arslan B, Turba UC, Matsumoto AH. Acute renal failure associated with percutaneous mechanical thrombectomy for iliocaval venous thrombosis. Semin Intervent Radiol 2007;24:288-95. [Crossref] [PubMed]
- Cynamon J, Stein EG, Dym RJ, et al. A new method for aggressive management of deep vein thrombosis: retrospective study of the power pulse technique. J Vasc Interv Radiol 2006;17:1043-9. [Crossref] [PubMed]
- Smith SJ, Behrens G, Sewall LE, et al. Vacuum-assisted thrombectomy device (AngioVac) in the management of symptomatic iliocaval thrombosis. J Vasc Interv Radiol 2014;25:425-30. [Crossref] [PubMed]
- Bozkurt A, Kırbaş İ, Kösehan D, et al. Pharmacomechanical thrombectomy in the management of deep vein thrombosis using the cleaner device: an initial single-center experience. Ann Vasc Surg 2015;29:670-4. [Crossref] [PubMed]
- Branco BC, Montero-Baker MF, Espinoza E, et al. Pharmacomechanical thrombolysis in the management of acute inferior vena cava filter occlusion using the Trellis-8 device. J Endovasc Ther 2015;22:99-104. [Crossref] [PubMed]
- Gloviczki P, Pairolero PC, Toomey BJ, et al. Reconstruction of large veins for nonmalignant venous occlusive disease. J Vasc Surg 1992;16:750-61. [Crossref] [PubMed]
- Venbrux AC, Mitchell SE, Savander SJ, et al. Long-term results with the use of metallic stents in the inferior vena cava for treatment of Budd-Chiari syndrome. J Vasc Interv Radiol 1994;5:411-6. [Crossref] [PubMed]
- Razavi MK, Hansch EC, Kee ST, et al. Chronically occluded inferior venae cavae: endovascular treatment. Radiology 2000;214:133-8. [Crossref] [PubMed]
- Funaki B. Inferior vena caval stenting. Semin Intervent Radiol 2004;21:347-9. [Crossref] [PubMed]
- Devcic Z, Techasith T, Banerjee A, et al. Technical and Anatomic Factors Influencing the Success of Inferior Vena Caval Stent Placement for Malignant Obstruction. J Vasc Interv Radiol 2016;27:1350-1360.e1. [Crossref] [PubMed]
- Guimarães M, Uflacker R, Schönholz C, et al. Stent migration complicating treatment of inferior vena cava stenosis after orthotopic liver transplantation. J Vasc Interv Radiol 2005;16:1247-52. [Crossref] [PubMed]
- Elson JD, Becker GJ, Wholey MH, et al. Vena caval and central venous stenoses: management with Palmaz balloon-expandable intraluminal stents. J Vasc Interv Radiol 1991;2:215-23. [Crossref] [PubMed]
- Saeed M, Knowles HJ Jr, Brems JJ, et al. Percutaneous retrieval of a large Palmaz stent from the pulmonary artery. J Vasc Interv Radiol 1993;4:811-4. [Crossref] [PubMed]
- Vedantham S, Vesely TM, Parti N, et al. Endovascular recanalization of the thrombosed filter-bearing inferior vena cava. J Vasc Interv Radiol 2003;14:893-903. [Crossref] [PubMed]
- Neglén P, Oglesbee M, Olivier J, et al. Stenting of chronically obstructed inferior vena cava filters. J Vasc Surg 2011;54:153-61. [Crossref] [PubMed]
- Kuehnl A, Schmidt M, Hornung HM, et al. Resection of malignant tumors invading the vena cava: perioperative complications and long-term follow-up. J Vasc Surg 2007;46:533-40. [Crossref] [PubMed]
- Nickel B, McClure T, Moriarty J. A Novel Technique for Endovascular Removal of Large Volume Right Atrial Tumor Thrombus. Cardiovasc Intervent Radiol 2015;38:1021-4. [Crossref] [PubMed]
- Sengodan P, Grewal H, Gandhi S, et al. Invasive hepatocellular carcinoma with recurrent pulmonary embolism: use of AngioVac cannula thrombectomy device for mechanical aspiration. J Invasive Cardiol 2014;26:E100-3. [PubMed]


