Cardiac tamponade due to the rupture of the coronary artery fistula
Introduction
Congenital coronary artery fistulas (CAFs) without symptoms are usually not considered as an indication for surgical treatment. We describe a rare case of cardiac tamponade secondary to spontaneous rupture of a CAF, which lead to death. An autopsy and pathological examination revealed that the CAF had histological abnormalities, which could have caused the rupture. This case report suggests that CAFs with extreme deformity should be considered for surgical treatment.
Case report
An 80-year-old man without known history of cardiac disease was admitted to our hospital for pre-surgical cardiovascular clearance. During a regular health check-up a month prior to admission, a chest X-ray showed an abnormal shadow in the left upper lobe. A bronchoendoscopy and CT scan revealed a lesion suggestive of lung cancer. He was scheduled for a left upper lobectomy, but the pre-surgical ECG showed decreased R waves in precordial leads and the echo cardiogram showed hypokinesis of antero-septal wall. He was therefore referred for further cardiovascular evaluation.
An examination on admission revealed a middle-sized man (height 159.7 cm, weight 63.1 kg, body temperature 36.6 °C, heart rate 64 beats/min, and blood pressure 150/64 mmHg). Auscultation of heart and lung were unremarkable. The patient’s CBC count was normal. ALT was slightly increased to 37 IU/L, CEA was increased to 6.1 ng/mL (normal 5.0 or less), and sialyl Lewis X-i was increased to 43 U/mL (normal 38 or less).
Cardiac catheterization and selected coronary angiography were performed. The left ventricular angiography was normal, but selective left coronary angiography showed a widened tortuous left circumflex coronary artery (LCX) with anastomosis to the left ventricle (LV). The appearance was consistent with a CAF originating from the LCX marginal vessel and terminating in LV (Figure 1). Selective right coronary angiography was normal. Right heart catheterization and an oximetry study did not show an oxygen saturation step-up from the right ventricle to the pulmonary artery. Such an oxygen step-up would be considered to be evidence of left-to-right heart shunt, and would be an indication for surgical treatment. In the absence of an oxygen step-up and since the patient did not show any symptoms of angina or heart failure, we concluded that the patient would not benefit from surgical treatment of the CAF.
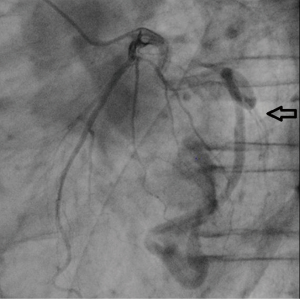
In the following month, a left upper lobectomy was performed. He had mild heart failure after the surgery, but recovered with diuretics. Ten days after the surgery he had a sudden chest pain. His echocardiogram showed rapid atrial fibrillation and ST depression at I, aVL, V3-6 leads. His chest X-ray showed remarkable cardiomegaly. An urgently performed chest CT scan and echocardiogram revealed an eccentric, large left sided pericardial effusion. He was diagnosed with cardiogenic shock secondary to cardiac tamponade. His diseased LCX was suspected to be the bleeding site. Emergent open-chest surgery was recommended, but his family refused surgical treatment. Two days later he died and autopsy was performed.
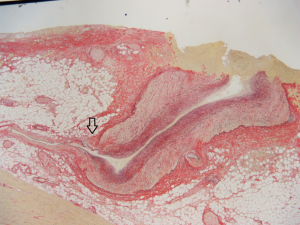
Autopsy revealed 450 mL of blood products in the pericardium. His LCX was widened and tortuous. It showed a tortuous course through the cardiac muscle layer and drained into the LV. This vessel was consistent with a CAF between LCX and LV. The CAF had some branches which did not terminate in LV but in the muscle layers, and the first branch had a rupture covered with a fresh blood clot. There was no sign of infection at the rupture site. The pathological examination also revealed that this branch vessel had irregular muscle layer. Specifically, partial defects of the muscle layer were observed at the ostium of smaller branches (Figure 2).
Discussion
In 1956 Alexander and Griffith reported that the incidence of coronary artery anomalies was 2.85 per thousand autopsies over a 10-year period (1). Likely secondary to increased coronary imaging with X-ray and CT angiography worldwide, recently coronary artery anomalies are found more frequently. In a continuous series of 1950 angiograms, Angelini et al. reported that the incidence of CAF was 0.87% (2). Summarizing their experience at the Cleveland Clinic, Yamanaka et al. describe among 126,595 patients who underwent coronary angiography in 28 years, coronary artery anomalies in 1,686 patients (1.3%) and CAFs in 225 patients (0.18%) (3). A report from Europe showed that the incidence of CAF was 0.13 among 14,708 patients underwent coronary arteriograms (4). A paper reviewing articles published between 2000 and 2010 revealed that unilateral fistulas originated from the left coronary artery (LCA) in 69% [Left Main Trunk (LMT) 7%, Left Anterior Descending (LAD) 42%, Left Circumflex (LCX) 20%] and right coronary artery (RCA) in 31% (5). CAF from LCX draining in LV was found in 4% among 243 CAFs (5).
The indications for operation are not established. Rittenhouse et al. describes a large shunt flow (average Qp/Qs=2.0) and symptoms of heart failure as justified indications for surgery, but also recommended operation for asymptomatic patients to prevent future symptoms or complications (6). Valente et al. stressed that the long-term complications of CAF closure may include coronary thrombosis, myocardial infarction, and cardiomyopathy (7).
Because our patient was asymptomatic until the age of 80, we did not recommend surgery for his CAF. We discussed if fistula rupture and cardiac tamponade could have been directly related to the lung cancer or the surgical procedure. However, the autopsy did not revealed inflammation related to the lung lesion, direct injury to the fistula or signs of infection. We therefore concluded that spontaneous rupture occured. Histology demonstrated muscle layer defects at the ostium of the fistula branches, which could have faciliated rupture in the setting of increased pressure in the fistula, specifically when LV pressures transmit in the fistula during systole.
There are a few case reports of ruptured CAF, resulting in cardiac tamponade. The causes of rupture are aneurysm (8,9), trauma (10,11), or spontaneous (12,13). A review article of published reports described that the risk factors for rupture of congenital aneurysmal fistula are female gender, saccular aneurysm, Asian ethnicity, origin of the aneurysmal fistulas from the left coronary artery and hypertension (14).
The acute presentation of our patient with chest pain and ST depression likely were secondary to myocardial ischemia in the setting of accelerated deterioration of cardiac function caused by the cardiac tamponade. Less likely, the rupture of CAF also might have lead to myocardial ischemia by stealing blood flow from LCX.
Conclusions
In summary, the optimal management of asymptomatic CAF is not completely established. Usually surgical treatment is avoided, but certain characteristic, including extreme tortuosity and aneurysmal dilatation, may be associated with increased risk of spontaneous rupture. We believe that CAFs with an extreme deformity should be considered for surgical treatment.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Alexander RW, Griffith GC. Anomalies of the Coronary Arteries and their Clinical Significance. Circulation 1956;14:800-5. [PubMed]
- Angelini P, Velasco JA, Flamm S. Coronary anomalies: incidence, pathophysiology, and clinical relevance. Circulation 2002;105:2449-54. [PubMed]
- Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn 1990;21:28-40. [PubMed]
- Gillebert C, Van Hoof R, Van de Werf F, et al. Coronary Artery Fistulas in an Adult Population. Eur Heart J 1986;7:437-43. [PubMed]
- Said SA. Current characteristics of congenital coronary artery fistulas in adults: A decade of global experience. World J Cardiol 2011;3:267-77. [PubMed]
- Rittenhouse EA, Doty DB, Ehrenhaft JL. Congenital coronary artery-cardiac chamber fistula. Review of operative management. Ann Thorac Surg 1975;20:468-85. [PubMed]
- Valente AM, Lock JE, Gauvreau K, et al. Predictors of long-term adverse outcomes in patients with congenital coronary artery fistulae. Circ Cardiovasc Interv 2010;3:134-9. [PubMed]
- Ryu JC, Choe YH, Park PW, et al. Cardiac tamponade due to a rupture of the coronary arteriovenous aneurysm--a case report. J Korean Med Sci 1997;12:143-5. [PubMed]
- Iwasawa Y, Kitamura Y, Higuma K, et al. Cardiac tamponade due to rupture of coronary artery fistulas with a giant aneurysm containing a free floating ball thrombus: a case report. J Cardiol 2007;50:71-6. [PubMed]
- Rossum A, Osborn L, Wernly J, et al. Cardiac stab wound resulting in a left anterior descending artery to left ventricular fistula with delayed pericardial tamponade. Cathet Cardiovasc Diagn 1994;31:283-5. [PubMed]
- Jeganathan R, Irwin G, Johnson PW, et al. Traumatic left anterior descending artery-to-pulmonary artery fistula with delayed pericardial tamponade. Ann Thorac Surg 2007;84:276-8. [PubMed]
- Gamma R, Seiler J, Moschovitis G, et al. Giant coronary artery fistula complicated by cardiac tamponade. Int J Cardiol 2006;107:413-4. [PubMed]
- Bauer HH, Allmendinger PD, Flaherty J, et al. Congenital coronary arteriovenous fistula: spontaneous rupture and cardiac tamponade. Ann Thorac Surg 1996;62:1521-3. [PubMed]
- Said SA, Schroeder-Tanka JM, Mulder BJ. Female gender and the risk of rupture of congenital aneurysmal fistula in adults. Congenit Heart Dis 2008;3:63-8. [PubMed]


