Clinical outcomes of PCSK9Is: a meta-analysis of randomized clinical trials
Introduction
Lipid lowering therapy with statins has been shown to reduce major adverse cardiovascular events (MACE) in numerous randomized controlled trials (RCTs) (1). High intensity statin therapy is recommended for secondary prevention of atherosclerotic cardiovascular disease (ASCVD) (2). A meta-analysis involving all the lipid lowering trials showed significant benefit with statin in preventing myocardial infarction (MI), stroke, and CV mortality (1). Studies involving non-statin agents like niacin have failed to show significant benefit (3). IMPROVE IT study showed that adding ezetimibe to statin therapy leads to reduction in MI and stroke but no effect on CV or all-cause mortality (4). Proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9Is) like evolocumab, alirocumab and the discontinued bococizumab cause marked reduction in LDL-cholesterol (LDL-C) when added to statin therapy (5-7). Phase III studies have demonstrated that these agents cause marked reduction in LDL-C with no significant adverse effects (5). We performed a meta-analysis to evaluate the effect of PCSK9Is use on outcomes.
Methods
This study was performed following procedures recommended by Cochrane collaboration (8) and is reported in accordance with recommendations set forth by Preferred Reported Items for Systematic Reviews and Meta-analyses (PRISMA) statement (8). Study endpoints included: major adverse cardiovascular events (MACE), myocardial infarction (MI), stroke, coronary revascularization, cardiovascular (CV) mortality and all-cause mortality.
Search methods
Embase, Medline and the Cochrane Central Register of Controlled Trials (CENTRAL) were searched for abstracts between January 2000 and March 2017. Different combinations of words were used for searching including: PCSK9Is, clinical outcomes of PCSK9Is, cardiovascular prevention and new cholesterol lowering agents.
Inclusion criteria included:
- Published data that at least reported one of the following clinical outcomes: MACE, MI, stroke, coronary revascularization, CV mortality and all-cause mortality;
- Randomized controlled trials;
- Double armed studies;
- Studies that reported clinical outcomes of PCSK9Is compared to control placebo group;
- Phase III trials.
Exclusion criteria included:
- Unpublished data;
- Single armed studies and case series;
- Studies not reporting the intended outcomes of this study;
- Trials in phase other than phase III;
- Studies comparing PCSK9Is to other medications rather than placebo.
Two reviewers (Rugheed Ghadban & Tariq Enezate) screened abstracts and titles to identify studies that qualified for review. We obtained the full manuscript for detailed review of studies meeting our inclusion criteria. Furthermore, the bibliographic references of identified review articles, meta-analyses and RCTs were screened, in order to identify RCTs not found by the electronic search.
Studies identification
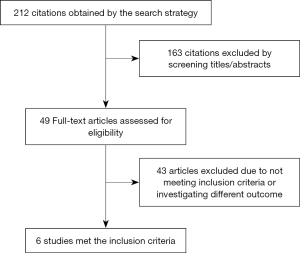
An English-language literature search was conducted using data sources mentioned in the study methods. Original papers irrespective of randomization were included. The initial search identified 212 citations of which 163 were excluded by reviewing titles and abstracts. We finally identified six RCTs from five original papers that satisfied the inclusion criteria of our study (Figure 1).
Data collection and extraction
Two reviewers (Rugheed Ghadban & Tariq Enezate) extracted data from the included RCTs based on pre-identified elements including the aim of each RCT, sample size, patient baseline characteristics, studies design, and type of endpoint measures including CV and all-cause mortality, MACE, MI, stroke, and coronary revascularization. The number of events in each study was documented when available. Table 1 depicts patients’ baseline characteristics as well as studies’ description and outcomes.
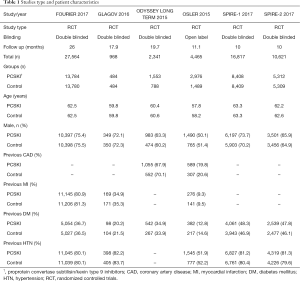
Full table
Risk of bias assessment
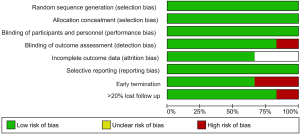
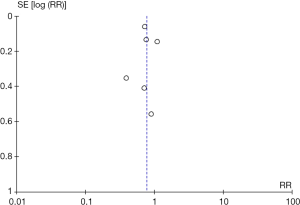
Methodological quality was defined as the control of bias assessed through the reported methods in each individual study using Cochrane Risk of Bias Tool to assess the quality of RCTs (9). Two independent reviewers (Rugheed Ghadban & Tariq Enezate) determined the quality of the included studies by examining risk of bias tool components. There was no evidence of high risk bias in regards to population selection, randomization, concealment allocation and baseline characteristics. However, there was a concern for inadequate follow up in ODYSSEY trial as more than 20% of patients were lost to follow up, also there was lack of blinding in OSLER, and attrition bias because of early termination of SPIRE 1 & 2 (5-7) (Table 1, Figure 2). No publication bias was found by noticing symmetry of the funnel plot (Figure 3) which provides a better display by spreading out the smaller studies on the bottom half of the plot, but has no bearing on the statistics.
Statistical analysis and data synthesis
Risk ratio (RR) was calculated using the inverse variance method for each outcome which allowed for pooling of similar outcomes. The average effects for the outcomes and 95% confidence intervals (CI) were obtained using a random effects model, as described by DerSimonian (10). The I2 statistic was used to assess the heterogeneity of treatment effect across studies that cannot be explained by random error or chance. A value of 50% or more indicates a significant heterogeneity that is the result of real differences in study populations, protocols, interventions and outcomes (10). A P value of 0.05 was used as a threshold for statistical significance for effect sizes. Analyses were conducted using features on RevMan version 5.3.5 (The Nordic Cochrane Center, Copenhagen, Denmark).
Methods for including both-armed zero events
In the case of zero events for an outcome in both groups simultaneously, we utilized a continuity factor of 1 due to the fact that RevMan software does not differentiate between “lack of data” (where no data reported) and “zero events” (which means that no events have occurred during the follow-up period). To solve this limitation, we added number 1 to all arms where event rates were zero in both experiment and control groups. This simple mathematical correction, will correct for zero events without affecting the accuracy of the statistical calculations and parameters to avoid computational errors (11).
Studies reporting no outcomes were not included in the analysis.
Results
A total of 60,029 patients (mean age 61 years, 73% were males) were included from six RCTs between year of 2015 and 2017 (5-7,12,13). All included patients had evidence of atherosclerotic disease, defined as history of coronary artery disease, non-hemorrhagic stroke and/or peripheral artery disease. Patients were comparable in both groups in terms of co-morbidities, atherosclerotic risk factors like hypertension and diabetes mellitus. The percentage of patients who were on statins was comparable between the two groups, although different statins at different doses were used. Non-statin therapies like ezetimibe were allowed.
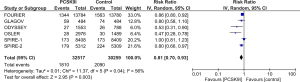
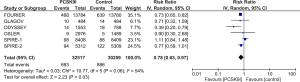
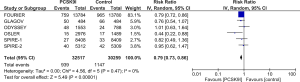
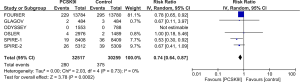
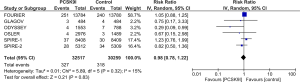
All studies reported incidence of MACE, MI and need for coronary revascularization. In comparison to control group, PCSK9Is group was associated with lower risk of MACE (RR =0.81, 95% CI, 0.70–0.93, P=0.003), MI (RR =0.78, 95% CI, 0.63–0.97, P=0.03) and need for coronary revascularization (RR =0.79, 95% CI, 0.73–0.86, P<0.00001) (Figures 4-6). Five out of six studies reported risk of stroke, there was lower risk of stroke in PCSK9Is group (RR =0.74, 95% CI, 0.64–0.87, P=0.0002) (Figure 7).
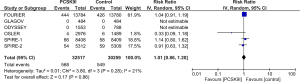
All studies reported CV mortality, while four out of six studies reported all-cause mortality. There was no significant difference observed between both groups in regards to CV mortality (RR =0.98, 95% CI, 0.78–1.22, P=0.83) or all-cause mortality (RR =1.01, 95% CI, 0.86–1.20, P=0.86) (Figures 8,9).
The random effects method was chosen as primary analysis because of its conservative summary estimate and incorporation between and within study variance. By using the fixed effect method, results remained unchanged. Sensitivity analyses were performed to assess the effects of selected measures of studies design on the pooled effect of PCSK9Is on clinical outcomes of atherosclerotic disease. The influence was estimated by performing a subgroup analysis and test for subgroup differences. The sub-analysis was performed after excluding the early terminated studies, the results remained consistent and unchanged from the overall analysis results on the six studies. When analysis was performed again after excluding the study with the largest population (i.e., FOURIER), results showed reduced risk for MACE and stroke in patients treated with PCSK9Is compared to control group, with no change in risk for MI, revascularization, CV mortality or all-cause mortality.
Discussion
PCSK9Is have shown to significantly reduce the level of LDL-C in preliminary trials which also demonstrated safety of this new generation of medications (14). A meta-analysis published in 2015 reported more than 50% reduction in LDL levels with no significant difference in adverse effects compared to control groups (14). However, the most recent AHA/ACC guidelines published in 2013 recommend no target LDL-C level to be achieved for primary or secondary prevention (2), therefore trials focusing on clinical outcomes have been done and some are ongoing. The question of whether further LDL-C reduction on top of that seen with statins is associated with improvement in clinical endpoints had to be answered. In 2016, the GLAGOV trial reported not only reduction in LDL-C levels, but also regression in plaque at 76 weeks in greater percentage of patients when adding evolocumab to statins. This was followed by the recently published FOURIER trial (12), which was powered for clinical outcomes and included more than 27,000 patients. They reported that the addition of evolocumab to statin significantly decreased the incidence of major CV events while there was no significant reduction in mortality compared to statin group.
We conducted a meta-analysis looking at the clinical outcomes of PCSK9Is. Many meta-analyses (15-20) were published comparing PCSK9Is to either placebo or other medications including (statins and ezetimibe), however to the best of our knowledge, our meta-analysis is the first on this topic to include all three RCTs available to date that are powered to assess clinical outcomes of interest (FOURIER and SPIRE 1 & 2) (7,12), while including only phase III trials. In addition to that, given the fact that statins are the standard of care in high risk patients, we opted to choose studies comparing PCSK9Is to placebo rather than statins, as it appears that PCSK9Is will play an important role as an addition rather than a replacement to the current standard of care therapy in patients at high risk for CVD. We did not aim to evaluate the effect on lowering LDL as this was studied extensively. We included six trials, three of which primarily reported the clinical outcomes of interest (7,12). Three out of six trials studied evolocumab (5,12,13), two studied bococizumab (7) and only one looked at alirocumab (6). There was a total of 92,004 patient-years of observation and follow up across all the trials included. We found a significant 19% reduction in MACE, 21% reduction in coronary revascularization and 22% reduction in MI, without a significant reduction in CV or all-cause mortality (P=0.86 and 0.86 respectively). This reduction in events is in line with the Cholesterol Treatment Trialists observation that there is a 22% reduction in MACE for every 39 mg/dL reduction in LDL-C (21). The FOURIER trial reported a 54 mg/dL decrease in LDL-C level on evolocumab and a 21% to 27% reduction in MI, stroke and coronary revascularization (12).
As previously mentioned, the SPIRE 1 & 2 trials were terminated early due to the decision of the manufacturer to discontinue the production of bococizumab. The high percentage of antidrug antibodies formed in patients receiving bococizumab is reported to have attenuated the LDL-C level lowering ability of the medication and maybe affected the clinical outcomes. Antidrug antibodies do not seem to be an issue with evolocumab or alirocumab (7,13,22). Therefore, a sub-analysis was performed excluding the SPIRE trials with no changes in results.
The significant reduction in MACE with no reduction in mortality, specifically CV related death might be due to the relatively short period of follow up in the included studies. There is a well documented delay between lowering LDL levels and the presence of positive clinical outcome in patients treated with statins and ezetimibe (4,17). Similarly, the OSLER and ODYSSEY LONG TERM trials, suggested that PCSK9Is significantly decreased the risk for MACE with progressively diverged cumulative incidence curves with time (5,6). Keeping in mind that the clinical outcomes were only exploratory in these two trials. Noteworthy, a meta-analysis of trials comparing intensive statin therapy with moderate intensity dosing also did not show a mortality benefit (23). Finally, the recently published FOURIER trial showed increase in risk reduction over time during the 2 years follow up period for combined end point. There was no effect on CV mortality of all causes mortality (12). Therefore, it is not yet known and remains to be determined if a longer duration of follow-up will show the difference in outcomes related to mortality. The results from the ongoing ODYSSEY OUTCOMES trial are awaited. This trial will include more than 18,000 patients who had acute coronary syndrome and will be followed for 4 years to assess long term morbidity and mortality benefit from adding PCSK9Is to statin (24).
Limitations
First of all, the analysis was performed on published results of the studies and not on patient level data.
Second, follow up for the included trials was relatively short with significant variation across trials.
The follow up was as short as 11 months in the OSLER trial (5), in addition to premature termination of the SPIRE trials (median follow up was 10 months) (7). On the other hand, the follow up in the FOURIER trial was 26 months (12). The short follow up in most of the trials, might have resulted an underestimation of the benefit in clinical outcomes including mortality. Short-term versus long-term outcome comparison was not done due to the fact that all studies, except for the FOURIER trial, had relatively short follow up period.
Also, while five out of six trials included were double-blinded, the OSLER trial which constituted more than 7% of total included patients was an open-label trial which could have affected event reporting rate (5). In addition, there was significant heterogeneity in some aspects of patients’ characteristics, most importantly in background lipid lowering therapy. The percentage of patients who were on high intensity statin therapy, which is the treatment recommended for high risk population (2), ranged from 26.7% to 91.7% of total studies’ patients (5,7). This is an important factor due to the fact that statin is known to increase the level of PCSK9, therefore PCSK9Is might have a synergistic effect when added to statin (14). Moreover, as previously mentioned, the outcomes looked at in this meta-analysis, were exploratory endpoints in three of the included trials, and primary endpoints in the other three, two of which were prematurely terminated (7,12). It is also important to mention that the discontinuation rate was high in some of the included trials. In the FOURIER trial, which is the largest study included in our analysis, the rate of discontinuation was up to 12.5% in both therapy and placebo arms (12). This rate was even higher in the ODYSSEY LONG TERM trial with up to 28.2% in the therapy group and 24.5% in the placebo group. Education about the benefits of taking the medication and injection site reactions might help with treatment adherence (6). Although PCSK9Is have shown to reduce LDL-C and improve clinical outcomes, the cost-effectiveness of these medications is yet to be determined. The availability of high potency statins, including atorvastatin and rosuvastatin as generic formulation has made them cost-effective (25,26).
Conclusions
PCSK9I use is associated with reduction in MACE, MI, coronary revascularization, and stroke but is not associated with reduction in all-cause mortality or CV mortality. PCSK9Is should be strongly considered to improve clinical outcomes in patients at high risk for atherosclerotic CVD. Future research is needed to help better assess long-term clinical outcomes including mortality.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267-78. [Crossref] [PubMed]
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S1-45. [Crossref] [PubMed]
- Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255-67. [Crossref] [PubMed]
- Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med 2015;372:2387-97. [Crossref] [PubMed]
- Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1500-9. [Crossref] [PubMed]
- Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1489-99. [Crossref] [PubMed]
- Ridker PM, Revkin J, Amarenco P, et al. Cardiovascular Efficacy and Safety of Bococizumab in High-Risk Patients. N Engl J Med 2017;376:1527-39. [Crossref] [PubMed]
- Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Updated March 2011.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Wells G, Shea B, O'Connell D, et al. The Newcasete-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. November 4, 2012 ed, 2012.
- Cheng J, Pullenayegum E, Marshall JK, et al. Impact of including or excluding both-armed zero-event studies on using standard meta-analysis methods for rare event outcome: a simulation study. BMJ Open 2016;6:e010983. [Crossref] [PubMed]
- Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med 2017;376:1713-22. [Crossref] [PubMed]
- Nicholls SJ, Puri R, Anderson T, et al. Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial. JAMA 2016;316:2373-84. [Crossref] [PubMed]
- Zhang XL, Zhu QQ, Zhu L, et al. Safety and efficacy of anti-PCSK9 antibodies: a meta-analysis of 25 randomized, controlled trials. BMC Med 2015;13:123. [Crossref] [PubMed]
- Vlachopoulos C, Terentes-Printzios D, Georgiopoulos G, et al. Prediction of cardiovascular events with levels of proprotein convertase subtilisin/kexin type 9: A systematic review and meta-analysis. Atherosclerosis 2016;252:50-60. [Crossref] [PubMed]
- Squizzato A, Suter MB, Nerone M, et al. PCSK9 inhibitors for treating dyslipidemia in patients at different cardiovascular risk: a systematic review and a meta-analysis. Intern Emerg Med 2017;12:1043-53. [Crossref] [PubMed]
- Schmidt AF, Pearce LS, Wilkins JT, et al. PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev 2017;4:CD011748. [PubMed]
- Sattar N, Preiss D, Robinson JG, et al. Lipid-lowering efficacy of the PCSK9 inhibitor evolocumab (AMG 145) in patients with type 2 diabetes: a meta-analysis of individual patient data. Lancet Diabetes Endocrinol 2016;4:403-10. [Crossref] [PubMed]
- Navarese EP, Kolodziejczak M, Schulze V, et al. Effects of Proprotein Convertase Subtilisin/Kexin Type 9 Antibodies in Adults With Hypercholesterolemia: A Systematic Review and Meta-analysis. Ann Intern Med 2015;163:40-51. [Crossref] [PubMed]
- Lipinski MJ, Benedetto U, Escarcega RO, et al. The impact of proprotein convertase subtilisin-kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network meta-analysis. Eur Heart J 2016;37:536-45. [Crossref] [PubMed]
- Cholesterol Treatment Trialists C, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670-81. [Crossref] [PubMed]
- Farnier M, Jones P, Severance R, et al. Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular-risk patients: The ODYSSEY OPTIONS II randomized trial. Atherosclerosis 2016;244:138-46. [Crossref] [PubMed]
- Cannon CP, Steinberg BA, Murphy SA, et al. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol 2006;48:438-45. [Crossref] [PubMed]
- Schwartz GG, Bessac L, Berdan LG, et al. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am Heart J 2014;168:682-9. [Crossref] [PubMed]
- Available online: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm498373.htm. 2016.
- Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-Year Risk Thresholds for Initiation of Statin Therapy for Primary Prevention of Cardiovascular Disease. JAMA 2015;314:142-50. [Crossref] [PubMed]