Rationale for catheter directed therapy in pulmonary embolism
Introduction
Pulmonary embolism (PE) occurs when there is obstruction of the pulmonary arteries due to thrombus, tumor, air or rarely foreign body. Most commonly, PE is due to venous thromboembolic disease arising from the legs which then travels to the pulmonary arteries. This can lead to significant strain on the right ventricle (RV) due to the increased afterload pressure. Furthermore, the thrombus prevents adequate oxygenation as deoxygenated blood is unable to reach the lung capillaries. PE remains a widespread, major cause of morbidity and mortality. Over 275,000 hospitalizations each year in the United States include the diagnosis of PE (1). The 3-month mortality in patients with massive PE is estimated to be as high as 50% with a mortality rate of 15% when accounting for all patients who present with PE (2).
Diagnostic evaluation of PE
When evaluating patients with suspected PE, the diagnostic workup starts with physical exam, EKG and a computed tomography with PE protocol (CTPE). CTPE is widely available and can be performed quickly. This gives precise anatomic detail with regards to the thrombus burden in terms of whether it is more central or peripheral within the pulmonary arteries in addition to laterality. Also, the CTPE study may offer an alternative diagnosis for the presenting symptoms such as pneumonia or pulmonary edema. In a patient with PE, additional useful information can be gained from the study such as whether there is evidence of right heart strain with a right ventricle/left ventricle (RV/LV) ratio >0.9, bowing of the septum, or reflux of contrast down the inferior vena cava suggesting right heart dysfunction. In a patient who is unable to receive iodinated contrast due to renal insufficiency or contrast allergy, a nuclear medicine VQ scan can be performed for the diagnosis of PE. In patients with massive or submassive PE, echocardiography is performed to assess right heart function. Specific physiologic detail such as the degree of right ventricular dyskinesis, RV/LV ratio and estimated pulmonary pressures can be gained. Laboratory exam includes cardiac markers such as troponin to assess for evidence of myocardial injury and brain natriuretic peptide (BNP) to evaluate for evidence of right ventricular strain.
Risk stratification
PE patients may present with a wide range of clinical manifestations. As such, PE is divided into three categories based on clinical severity (3). Massive PE or high risk PE occurs in about 5% of all PE patients and is defined as hemodynamic instability with a systolic blood pressure <90 mmHg lasting greater than 15 minutes. These patients are at high risk for death—up to 65% in-hospital mortality in patients requiring cardiopulmonary resuscitation (4,5). In these cases, guidelines support the use of systemic thrombolysis for treatment and catheter directed therapies (CDTs) in some patients. Submassive PE or intermediate PE patients represent about 25% of all PE patients (6). These patients have evidence of right ventricular strain along with myocardial injury without systemic hypotension. While hemodynamically stable, these patients fall into a range of minimal symptoms to those requiring resuscitative efforts such as supplemental oxygenation or even intubation to maintain adequate oxygenation. These patients are at risk for increased morbidity in the form of shortness of breath with oxygen requirements, clinical deterioration, longer hospitalization and longer recovery. Furthermore, they have a mortality rate in the 2–3% range (7). The treatment of choice in submassive PE is less clear. Considerations would include anticoagulation, systemic thrombolysis or CDTs. The last category of PE patients is the low risk patients. These patients have no evidence of heart dysfunction and are hemodynamically stable. They are treated with anticoagulation with no need for additional therapies with a very low associated mortality.
Treatment of massive PE
In patients with massive PE (systolic BP <90), the cause of death is often cardiogenic shock related to increased afterload pressure from the obstructive thrombus in the pulmonary artery. In addition to the physical obstruction related to the thrombus, hypoxemic vasoconstrictors are released further increasing the degree of pulmonary vascular resistance. The acute increase in the right ventricular pressure places strain on the RV which can lead to significant dysfunction and cardiogenic shock. The decreased right ventricular output results in lower left ventricular pre-load volumes with subsequent systemic hypotension. In addition, the RV bowing into the LV can decrease the pre-load of the LV. Finally, the increased wall stress in the right ventricular system along with the increased demand can lead to ischemia of the right ventricular wall (8). In this setting, it is critical to decrease the thrombus burden quickly to decrease the afterload. Standard anticoagulation with heparin will decrease the chance of thrombus formation or propagation, but does little to decrease thrombus burden in the acute setting. In contrast, thrombolytic agents can be administered via a peripheral IV which actively dissolves thrombus by degradation of fibrin molecules. A generally accepted and FDA approved protocol is the administration of 100 mg of alteplase via a peripheral IV over a 2-hour period (9).
Systemic thrombolytics in massive PE
The mortality reduction related to the use of systemic thrombolytics in massive PE has been demonstrated. A retrospective analysis of a nation-wide database evaluated patients with massive PE treated with systemic thrombolytics versus standard anticoagulation alone. The all-cause in-hospital fatality rate was 15% in the thrombolytic group compared to 47% in those without thrombolytic therapy (P<0.0001) (10). In a meta-analysis comparing thrombolytics to anticoagulation alone, a subgroup analysis was performed in five trials that differentiated patients with massive (hemodynamic instability) PE and found a significant reduction in death in patients who received thrombolytics versus anticoagulation alone (9.4% vs. 19.0%) (11). The American Heart Association recommendation states that systemic fibrinolysis is reasonable for patients with massive acute PE and acceptable risk of bleeding complications (Class IIa; Level of Evidence B) (3). In addition, the American College of Chest Physicians (ACCP) guidelines suggest that patients with acute massive PE (systolic BP <90 mmHg for 15 minutes) and without a high bleeding risk can be treated with thrombolytic therapy (Grade 2B) (12). However, the use of systemic thrombolytic therapies must be weighed against the increased risk of major hemorrhage (discussed in detail below). Estimates regarding the risk of bleeding from systemic thrombolytics range anywhere from 5% to 20%.
CDTs in massive PE
CDTs for the treatment of massive PE have been used for over two decades. These therapies have the advantage of immediately decreasing the proximal thrombus burden thereby lowering the afterload pressure exerted on the RV and ultimately improving cardiac output. Several endovascular techniques exist for CDT in the acute setting for massive PE. One is the use of maceration to disrupt the thrombus in the proximal pulmonary arteries to effectively send thrombotic fragments into the distal branches thereby decreasing the proximal obstruction and strain on the RV. A number of different techniques are available such as twirling a pigtail catheter within the clot, angioplasty or simple wire agitation (see Table 1). Another technique is suction embolectomy, essentially using a catheter to aspirate the thrombus. Numerous devices are available which function on this principle. Finally, rheolytic thrombectomy catheters can be used for thrombus removal. These devices use a high flow saline jet stream with resultant low pressure within a catheter allowing thrombus aspiration (18). All of these techniques can be used in conjunction with a thrombolytic agent allowing direct administration into the pulmonary arteries or ideally, into the thrombus. One advantage of intrathrombus delivery is that it allows a greater surface area of thrombus to be in direct contact with the thrombolytic. Due to the direct delivery, doses of the thrombolytic can be much lower compared to systemic therapies.
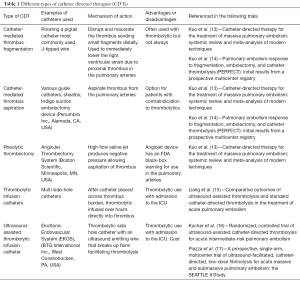
Full table
One meta-analysis looking at CDT for massive PE in 594 patients showed a pooled clinical success rate of 86.5% (13). This meta-analysis included all types of interventions including maceration (most commonly twirling a pigtail catheter in the pulmonary artery), suction embolectomy and rheolytic catheter embolectomy. About 1/3 of patients did not receive a thrombolytic agent and utilized only mechanical disruption. The rheolytic AngioJet device (Boston Scientific, Minneapolis, MN, USA) was associated with a high number of complications, including five procedure-related deaths. As such, the FDA has issued a black box warning on the label to alert users to the potential complications related to the use of this device in the pulmonary arteries.
CDT for the treatment of massive PE has been shown to be safe. A 7.9% minor procedural complication rate and a 2.4% major complication rate were found in the meta-analysis of CDT by Kuo and colleagues (13). This compares to an approximately 20% major bleeding rate with the use of systemic thrombolytics (ICOPER study) (2). One advantage of CDT in massive PE patients is the ability to decrease thrombus burden immediately using physical disruption and/or embolectomy. In addition, CDT can be used in patients who have a contraindication to thrombolytic therapy. If the patient is a candidate for thrombolysis, a much lower dose can be used using CDT versus systemic therapy. The AHA recommendations state that depending on local expertise, either catheter embolectomy or fragmentation is reasonable for patients with massive PE and contraindications to fibrinolysis. Catheter embolectomy and fragmentation or surgical embolectomy is reasonable for patients with massive PE who remain unstable after receiving systemic fibrinolysis (Class IIa; Level of evidence C) (3). The ACCP guidelines state in patients with acute PE with hypotension and who have (I) a high bleeding risk, (II) failed systemic thrombolysis, or (III) shock that is likely to cause death before systemic thrombolysis can take effect, if appropriate expertise and resources are available, catheter-assisted thrombus removal is suggested over no such intervention (Grade 2C) (12).
Treatment of submassive PE
While the use of thrombolytic therapy may be justified in massive PE if a patient has an acceptable bleeding risk according to the AHA and ACCP guidelines, the use of systemic thrombolytic therapy in patients with submassive PE is generally not recommended. The exception is in select patients who demonstrate cardiopulmonary deterioration or develop hypotension after the initiation of anticoagulation. Systemic thrombolytics have been shown to improve cardiopulmonary status in patients with submassive PE, however, the limitation lies largely with the increased risk of major bleeding. CDT has the advantage over systemic therapies of using a lower dose of thrombolytics thereby lowering the risk of bleeding. Initial studies looking at catheter based therapies have shown significant improvement in short term endpoints with a very good safety profile. The following will highlight some of the larger studies evaluating treatment options for submassive PE looking at short and long term result along with complication rates associated with these therapies.
Short term efficacy
There have been prospective studies evaluating the use of systemic thrombolytics in patients with submassive PE. The PEITHO study was a randomized, double blind study in 1,005 submassive PE patients comparing the thrombolytic agent tenecteplase with heparin to a placebo with heparin (19). Death or hemodynamic decompensation occurred within 7 days from randomization in 2.6% of the tenecteplase group compared to 5.6% in the placebo group. However, stroke (mostly hemorrhagic) occurred in 2.4% in the tenecteplase group compared to 0.2% in the placebo group. The MOPETT study was a randomized controlled trial of 121 patients who were divided into a ‘safe dose’ thrombolytic (half of the full dose) with anticoagulation and an anticoagulation group only (20). Benefits were noted at 48 hours with a more marked reduction in the pulmonary artery pressures in the thrombolytic group compared to the anticoagulation group only (16 vs. 10 mmHg reduction, P<0.001). Additionally, the days of hospitalization were shorter in the thrombolytic group compared to the anticoagulation group (2.2 vs. 4.9, P<0.001). These studies did show short term benefit to the use of thrombolytics in patients with submassive PE albeit with an increased risk of bleeding.
Given the benefit demonstrated with the use of systemic thrombolytics, the question arises on whether CDT can offer the same benefit without the increased risk of bleeding. There have been a few studies looking at CDT therapy in patients with submassive PE. The ULTIMA trial was a multicenter randomized, controlled trial in 59 patients comparing CDT (specifically ultrasound-assisted catheter directed thrombolysis) to anticoagulation alone (16). The primary outcome was difference in the RV/LV ratio at 24 hours after the initiation of therapy. In the CDT group, the mean decrease was 0.30±0.20 compared to a decreased ratio of 0.03±0.16 (P<0.001) in the heparin only group. The SEATTLE II trial was a prospective, single-arm, multicenter trial evaluating 150 patients (119/150 with submassive PE) who underwent CDT with ultrasound-assisted catheter directed thrombolysis. The primary efficacy outcome was the change in the RV/LV ratio based on CT imaging. The study found a reduction in the mean RV/LV ratio from 1.55 to 1.13; mean difference −0.42; P<0.0001 within 48 hours of therapy initiation. The mean pulmonary artery systolic pressure decreased from 51.4 to 36.9 mmHg; P<0.0001. Furthermore, the modified Miller Index score decreased post-procedure from 22.5 to 15.8; (P<0.0001) (17). These studies both used ultrasound-assisted thrombolysis (EKOS, BTG International Inc., West Conshohocken, PA, USA) in which a wire emits ultrasound waves which disrupt fibrin allowing lower doses of the thrombolytic. Conventional multi-side hole thrombolytic catheters also have been studied. A retrospective review comparing ultrasound assisted thrombolysis to standard thrombolysis via a multi-side hole catheter (without an ultrasound emitting wire) was performed and found no significant difference in the clinical or hemodynamic outcomes (15). The Pulmonary Embolism Response to Fragmentation, Embolectomy and Catheter Thrombolysis (PERFECT) study was a prospective multicenter registry evaluating 100 consecutive patients receiving a variety of CDT for PE (73/100 submassive). CDT included mechanical, pharmacomechanical and catheter directed thrombolysis. Clinical success was defined as meeting three criteria: stability of hemodynamics, improvement in pulmonary hypertension, right-sided heart strain, or both and survival to hospital discharge. In the submassive PE group, clinical success was achieved in 71/73 patients (97.3%; 95% CI, 90.5–99.7%). Of all PE patients who had invasive pulmonary artery pressure measurements performed, 78/92 showed significant improvement in pulmonary artery pressures (51.2±14.1 mmHg lowered to 37.2±15.8 mmHg post P<0.0001) (14).
Long term efficacy
While in-hospital short term morbidity and mortality are important in deciding which patients should be treated with more invasive therapies, it is also important to consider the long term sequelae of PE. The ‘post PE syndrome’ refers to a symptomatology that can persist years following an acute PE. These patients may have functional limitations or decreased quality of life as a result of acute PE. About 10–30% of patients may have abnormal pulmonary artery pressures and right ventricular function despite adequate anticoagulation (21). The long term effects of PE on quality of life have been demonstrated. In a study of 392 acute PE patients, the SF-36 was completed at least one year following PE and compared to population norms. This found significant decrease in physical functioning, social functioning, physical role limitations and general health perceptions measures (P<0.001) (22). One prospective trial evaluated 109 patients without other significant co-morbidities who presented with PE. These patients underwent a baseline and 6-month echocardiogram. In addition, at 6 months, a 6 minute walk distance (6MWD) and a quality of life survey was completed. They found 41% of previously healthy patients had either an abnormal echocardiogram, a New York Heart Association (NYHA) score >II or a 6MWD <330 m at 6 months (23). However, the long term sequelae depend on the echocardiogram findings at presentation. If a patient has no evidence of the right heart strain on presentation, the likelihood of developing pulmonary hypertension is low (24).
Chronic thromboembolic pulmonary hypertension (CTPH) represents the extreme form on this spectrum and may be seen in up to 4% of patients following an acute PE. These patients have persistently elevated pulmonary artery pressures and clinically suffer from chronic dyspnea on exertion. One prospective study found the cumulative incidence of CTPH to be 3.8% at 2 years (25). Risk factors for CTPH included recurrent PE, larger PE and young age.
Given the potential long term sequelae from PE, particularly in those patients with right heart strain, the next question is can more aggressive therapy be performed in the acute setting to decrease the long term effects of PE. The MOPETT study evaluated long term effects of systemic thrombolytics compared to standard anticoagulation alone using pulmonary hypertension as a primary endpoint. The study included submassive PE patients who were prospectively randomized into the two groups. Echocardiograms were performed at baseline and at mean follow up of 28 months. Pulmonary hypertension (defined as pulmonary pressure greater or equal to 40 mmHg) was present in 16% of the thrombolytic group compared to 57% in the anticoagulation group. The mean decrease in pulmonary pressure at 28 months was 22 mmHg in the thrombolytic group vs. 8 mmHg in the anticoagulation group (P<0.001) (20). Another prospective study evaluated 72 patients with submassive PE and divided them into two groups—a thrombolytic group with anticoagulation verses anticoagulation only. Patients underwent echocardiography at 24, 48 and 72 hours and at 3 and 6 months. The thrombolysis group showed a significant early improvement of RV function compared to the heparin group. This improvement was seen at the 6-month interval with a statistically significant decreased pulmonary pressure from baseline to 6 months (40 mmHg decrease in the thrombolytic group vs. the 35 mmHg heparin group decrease; P<0.0001) (26). Another prospective randomized study in patients with submassive PE divided patients into a thrombolytic group and placebo and evaluated outcomes at 3 months using the SF-36 survey. Patients who received the thrombolytic showed better outcomes compared to the placebo group. Statistical significance was present on the role physical, general health and physical component summary (27).
Safety of CDTs
While the benefits of systemic thrombolytics in massive and submassive PE have been shown in numerous studies, these benefits come at a risk of bleeding. The risks can range from small puncture site hematomas to life threatening bleeds with hypotension or intracranial hemorrhage. The risk of major hemorrhage related to systemic thrombolysis has been shown to be as high as 20%. One study evaluated 104 patients who received alteplase 100 mg for acute PE and found a 19.2% rate of major bleeding. Of the patients who had major bleeding, the principal site of bleeding was unknown in 9 (45%), GI tract in 6 (30%), retroperitoneum in 3 (15%) and intracranial in 1 (5%) (9). The ICOPER registry found a 21.7% rate of major bleeding in patients who received thrombolytics with a 3.0% rate of intracranial bleeding (2). In a meta-analysis evaluating trials that compared thrombolytics to anticoagulation only, thrombolytics were associated with an increased risk of major bleeding (9.1% vs. 6.1%) and a significant increased risk of non-major bleeding (22.7% vs. 10.0%) (11).
In theory, CDTs allow a lower dose of thrombolytic to be used since there is a local targeted delivery directly into the thrombus. Systemic thrombolytics also have the disadvantage of preferential flow to non-obstructed vessels—away from the thrombus burden thereby decreasing contact with the thrombus. CDT also offers the advantage of some degree of thrombus disruption either by ultrasound waves or mechanical disruption using wires, catheters or embolectomy devices.
The safety profile for CDT in PE has been favorable. The PERFECT registry evaluated 100 patients with differing types of CDT and found no major procedural related complications, major hemorrhage or hemorrhagic strokes. There were 13 minor bleeding events of which 6 were access site related. None of the minor events required a blood transfusion (14). The SEATTLE II study showed major bleeding (defined as both moderate bleeding and severe/life threatening bleeding) in 10% of all patients. Of these, one patient had a GUSTO (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) severe/life threatening hemorrhage requiring vasopressor support. This was a groin hematoma. There were no intracranial bleeds. The remainder of the major bleeds were categorized as moderate which were defined as bleeds requiring a blood transfusion without evidence of hemodynamic compromise (17). The ULTIMA trial showed no major bleeding complications in the 30 patients receiving ultrasound assisted thrombolysis. There were 3 patients with minor bleeding (one access site hematoma and two patients with transient hemoptysis) none of which required blood transfusion (16). A meta-analysis looking at CDT in submassive PE found low pooled rates of adverse events with very low major and minor bleeding complications (28). Another meta-analysis evaluating CDT for massive PE showed a favorable safety profile; the pooled risks of minor and major procedural complications were 7.9% and 2.4% respectively (13).
Future trends for CDT in PE
In general, there is clinical acceptance for the use of CDT in massive PE particularly at centers with expertise in these techniques. Systemic thrombolytics can be considered as well for massive PE. At our institution, it is rare for systemic thrombolytics to be administered for submassive PE. After a thorough evaluation of the risks and benefits, we do consider CDT for submassive PE. Within the category of submassive PE patients, we feel there may be subset of patients who will likely benefit from CDT more than others. These higher risk patients are most likely to gain benefit in the short and long term. In our practice all cases are discussed in a multidisciplinary fashion with vascular medicine, the intensive care unit and referring service. Many hospitals have developed pulmonary embolism response teams (PERT) to better address these issues, allow open dialogue and gain experience with this complex patient population. When evaluating the submassive PE patient, we look for evidence of right heart strain on echocardiography, elevated troponins and BNP and at least some clinical signs of respiratory compromise such as shortness of breath or poor oxygenation. Patients should not be at high risk for bleeding. While scoring systems such as the Pulmonary Embolism Severity Index (PESI) or the simplified version (sPESI) may be useful for risk stratification, we tend to use the AHA stratification definitions of massive, submassive and low risk along with their associated mortality risk (29).
As reviewed, short and long term benefits of systemic thrombolysis have been demonstrated in multiple studies, albeit with an increased risk of bleeding. Additionally, the short term benefits of CDT in massive and submassive PE have shown promising results in the form of improved RV function, decreased pulmonary artery pressures and decreased hospitalization days compared to those patients receiving anticoagulation only. There have been numerous retrospective reviews demonstrating the short term benefits of CDTs in massive and submassive PE (30-34). However, the long term benefits of CDT in the submassive population have not been evaluated in a prospective study. The impact of CDT on preventing the so called ‘post PE syndrome’ 2–3 years after the acute PE event has not yet been studied. A prospective study in high risk submassive PE patients comparing anticoagulation alone to CDT with anticoagulation utilizing both short and long term endpoints would be ideal.
Conclusions
CDT in the treatment of PE is rapidly evolving. Numerous studies have shown the short term benefits of systemic thrombolysis in patients with PE. Furthermore, the long term benefits of systemic thrombolysis have been suggested. However, systemic thrombolysis comes at a risk of increased bleeding. At the same time, new interventional devices and techniques have been developed allowing a minimally invasive approach to treating PE patients. Many CDT techniques employ the use of thrombolytics, however, the dose utilized is generally about one third the dose given for systemic thrombolytics. As such, the bleeding risks associated with CDT, while still present, tend to be lower compared to systemic thrombolytics. The reduction in pulmonary pressures and right ventricular strain has been demonstrated with CDT with an excellent safety profile. Questions still remain regarding the role of CDT in submassive PE and accurately identifying which patients will benefit the most. Further investigative work is clearly necessary to help fine tune the role of CDT in PE.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Centers for Disease Control and Prevention (CDC). Venous thromboembolism in adult hospitalizations - United States, 2007-2009. MMWR Morb Mortal Wkly Rep 2012;61:401-4. [PubMed]
- Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999;353:1386-9. [Crossref] [PubMed]
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011;123:1788-830. [Crossref] [PubMed]
- Kasper W, Konstantinides S, Geibel A, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol 1997;30:1165-71. [Crossref] [PubMed]
- Konstantinides SV. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014;35:3145-6. [PubMed]
- Kearon C. Natural history of venous thromboembolism. Circulation 2003;107:I22-30. [Crossref] [PubMed]
- Konstantinides S, Geibel A, Heusel G, et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med 2002;347:1143-50. [Crossref] [PubMed]
- Piazza G, Goldhaber SZ. Management of submassive pulmonary embolism. Circulation 2010;122:1124-9. [Crossref] [PubMed]
- Fiumara K, Kucher N, Fanikos J, et al. Predictors of major hemorrhage following fibrinolysis for acute pulmonary embolism. Am J Cardiol 2006;97:127-9. [Crossref] [PubMed]
- Stein PD, Matta F. Thrombolytic therapy in unstable patients with acute pulmonary embolism: saves lives but underused. Am J Med 2012;125:465-70. [Crossref] [PubMed]
- Wan S, Quinlan DJ, Agnelli G, et al. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation 2004;110:744-9. [Crossref] [PubMed]
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016;149:315-52. [Crossref] [PubMed]
- Kuo WT, Gould MK, Louie JD, et al. Catheter-directed therapy for the treatment of massive pulmonary embolism: systematic review and meta-analysis of modern techniques. J Vasc Interv Radiol 2009;20:1431-40. [Crossref] [PubMed]
- Kuo WT, Banerjee A, Kim PS, et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT): Initial Results From a Prospective Multicenter Registry. Chest 2015;148:667-73. [Crossref] [PubMed]
- Liang NL, Avgerinos ED, Marone LK, et al. Comparative Outcomes of Ultrasound-Assisted Thrombolysis and Standard Catheter-Directed Thrombolysis in the Treatment of Acute Pulmonary Embolism. Vasc Endovascular Surg 2016;50:405-10. [Crossref] [PubMed]
- Kucher N, Boekstegers P, Muller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014;129:479-86. [Crossref] [PubMed]
- Piazza G, Hohlfelder B, Jaff MR, et al. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism: The SEATTLE II Study. JACC Cardiovasc Interv 2015;8:1382-92. [Crossref] [PubMed]
- Zarghouni M, Charles HW, Maldonado TS, et al. Catheter-directed interventions for pulmonary embolism. Cardiovasc Diagn Ther 2016;6:651-61. [Crossref] [PubMed]
- Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014;370:1402-11. [Crossref] [PubMed]
- Sharifi M, Bay C, Skrocki L, et al. Moderate pulmonary embolism treated with thrombolysis (from the "MOPETT" Trial). Am J Cardiol 2013;111:273-7. [Crossref] [PubMed]
- Klok FA, van der Hulle T, den Exter PL, et al. The post-PE syndrome: a new concept for chronic complications of pulmonary embolism. Blood Rev 2014;28:221-6. [Crossref] [PubMed]
- Klok FA, van Kralingen KW, van Dijk AP, et al. Quality of life in long-term survivors of acute pulmonary embolism. Chest 2010;138:1432-40. [Crossref] [PubMed]
- Stevinson BG, Hernandez-Nino J, Rose G, et al. Echocardiographic and functional cardiopulmonary problems 6 months after first-time pulmonary embolism in previously healthy patients. Eur Heart J 2007;28:2517-24. [Crossref] [PubMed]
- Grifoni S, Vanni S, Magazzini S, et al. Association of persistent right ventricular dysfunction at hospital discharge after acute pulmonary embolism with recurrent thromboembolic events. Arch Intern Med 2006;166:2151-6. [Crossref] [PubMed]
- Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004;350:2257-64. [Crossref] [PubMed]
- Fasullo S, Scalzo S, Maringhini G, et al. Six-month echocardiographic study in patients with submassive pulmonary embolism and right ventricle dysfunction: comparison of thrombolysis with heparin. Am J Med Sci 2011;341:33-9. [Crossref] [PubMed]
- Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost 2014;12:459-68. [Crossref] [PubMed]
- Lou BH, Wang LH, Chen Y. A meta-analysis of efficacy and safety of catheter-directed interventions in submassive pulmonary embolism. Eur Rev Med Pharmacol Sci 2017;21:184-98. [PubMed]
- Sista AK, Kuo WT, Schiebler M, et al. Stratification, Imaging, and Management of Acute Massive and Submassive Pulmonary Embolism. Radiology 2017;284:5-24. [Crossref] [PubMed]
- Akin H, Al-Jubouri M, Assi Z, et al. Catheter-directed thrombolytic intervention is effective for patients with massive and submassive pulmonary embolism. Ann Vasc Surg 2014;28:1589-94. [Crossref] [PubMed]
- Fuller TJ, Paprzycki CM, Zubair MH, et al. Initial Experiences with Endovascular Management of Submassive Pulmonary Embolism: Is It Safe? Ann Vasc Surg 2017;38:158-63. [Crossref] [PubMed]
- Nykamp M, VandenHull A, Remund T, et al. Safety and efficacy of ultrasound-accelerated catheter-directed lytic therapy in acute pulmonary embolism with and without hemodynamic instability. J Vasc Surg Venous Lymphat Disord 2015;3:251-7. [Crossref] [PubMed]
- Avgerinos ED, Liang NL, El-Shazly OM, et al. Improved early right ventricular function recovery but increased complications with catheter-directed interventions compared with anticoagulation alone for submassive pulmonary embolism. J Vasc Surg Venous Lymphat Disord 2016;4:268-75. [Crossref] [PubMed]
- Lee KA, Cha A, Kumar MH, et al. Catheter-directed, ultrasound-assisted thrombolysis is a safe and effective treatment for pulmonary embolism, even in high-risk patients. J Vasc Surg Venous Lymphat Disord 2017;5:165-70. [Crossref] [PubMed]


