Hemodialysis access thrombosis
Introduction
Approximately 430,000 patients in the United States are dependent on hemodialysis (HD) (1). Options for HD access include catheters, arteriovenous grafts (AVGs) and arteriovenous fistulas (AVFs). AVG thrombosis occurs approximately 0.5–2.0 times per year and AVF thrombosis occurs 0.1 to 0.5 times per year (2). AV access thrombosis occasionally leads to multiple missed dialysis sessions, inpatient admission and placement of a temporary dialysis catheter. Access thrombosis accounts for 65–85% of cases of permanent access loss (2). After considering potential contraindications, endovascular declot should be performed. Various approaches include pulse spray aided pharmacomechanical thrombolysis, mechanical clot maceration and the lyse-and-wait technique (3-5).
Monitoring and surveillance
Monitoring and surveillance are employed in order to identify a dialysis access at risk of thrombosis with the goal of intervening before thrombosis occurs (6,7). The efficacy of monitoring and surveillance in preventing access thrombosis and prolonging the life of the access is uncertain (8,9).
Monitoring consists of focused physical examination of the access and review of data gathered during the normal course of dialysis such as Kt/V, serum potassium, pump pressures and problems with access cannulation (e.g., prolonged bleeding after needle removal) (6). Physical examination, performed at least monthly by a qualified practitioner, includes looking at, feeling and listening to the access to identify possible stenoses that place the access at risk of thrombosis. Signs of outflow stenosis include development of aneurysmal dilation of the access, abnormally increased pulsatility, a discontinuous thrill and a high-pitched bruit in the outflow vein. Signs of inflow stenosis include a flat fistula, poor turgor or a weak bruit (10). Kt/V quantifies dialysis adequacy. K represents the clearance of urea, t represents the dialysis time and V represents the volume of distribution of urea. Low Kt/V denotes inadequate dialysis, which adversely affects the patient’s quality of life and is associated with lower survival (11). A low Kt/V may indicate poor access flow which places the access at risk for subsequent thrombosis (12). A Kt/V of <1.2 or an interval decrease by more than 0.20 should prompt intervention both to increase dialysis adequacy as well as forestall potential access thrombosis (13). Prolonged bleeding after needle withdrawal is a common sign of outflow stenosis. Excessively negative pre-pump arterial pressure is a sign of inflow stenosis.
Surveillance is the periodic employment of specialized instrument based testing. Examples include access flow measurements (Qa), static venous pressure measurements, quantification of recirculation and Duplex ultrasound (6).
Access flow measurement is the most reliable and validated surveillance tool (14). AVGs are at risk of thrombosis when Qa falls below 500 mL/min. AVFs are at risk of thrombosis when Qa is less than 300 mL/min. The main reason AVGs thrombose at higher flow rates than AVFs is that they lack an endothelium (15). Falling Qa is highly predictive of subsequent access thrombosis; one study showed a near 14-fold increase in access thrombosis when Qa dropped to 65% of baseline (16).
Static venous pressure measurements are useful in AVGs where the site of stenosis predictably occurs just downstream from the graft-to-vein anastomosis. Static venous pressure measurements in AVFs are of lower utility in predicting access thrombosis for multiple reasons. For one, AVFs often have stenosis involving the inflow. Additionally collateral vessels may develop and enlarge, dissipating the increased intra-access pressures in the setting of outflow stenosis. An access to systemic pressure ratio less than 0.4 is normal; a ratio greater than 0.5 should trigger evaluation (17).
Recirculation occurs when blood that has just been through the dialysis machine returns back into the dialysis machine rather than flowing towards the right atrium. This can occur with both with inflow and outflow stenoses. By definition recirculation can only occur when Qa is less than pump flow rate (Qb). When recirculation is due to outflow stenosis, it occurs because the path of least resistance is retrograde, through the AV access (i.e., towards the feeding artery) and into the arterial needle, rather than antegrade (i.e., through the outflow stenosis). When recirculation occurs secondary to inflow stenosis it is because the inflow is unable to support the amount of flow required to maintain the set Qb; the arterial needle has to “borrow” blood returning into the access via the venous needle to maintain the flow rate demanded by the pump (13). Recirculation is an ineffective surveillance technique. First, it is only an indirect measure of Qa, which can be measured more precisely by other methods. Second, it is insensitive in identifying an AVG at risk of thrombosis. This because Qb is typically set at 300 mL/min and recirculation will only occur when Qa is less than this set Qb. An AVG is at risk of thrombosis when flow rates are less than 500 mL/min. Additionally, stenosis between the two needles cannot be detected by measuring recirculation. Finally, recirculation can occur when there is accidental needle reversal, making it a non-specific finding.
Ultrasound can be used as a surveillance tool. A >50% stenosis is likely when there is a focally elevated peak systolic velocity of >400 cm/s or a local peak systolic velocity ratio of >2.5. Gray scale and color Doppler ultrasound can also directly show a focal lumen diameter reduction. Caution should be taken when applying these thresholds at the arterial anastomosis where the normal turbulent flow leads to elevated peak systolic velocities. Ultrasound can also be used to measure flow volumes within the access (18).
Controversy surrounds the efficacy of surveillance. Studies have shown that surveillance of AVGs increases the discovery of stenoses and the number of interventions performed but does not seem to prolong their lifespan (9). In contrast, studies have shown surveillance for AVFs decreases the frequency of thrombosis and prolongs access survival (8).
Systemic anti-platelet/anticoagulation therapy
Systemic anticoagulants and anti-platelet therapy have been used to prevent access thrombosis with minimal success. One meta-analysis of anti-platelet therapy showed a decrease in early (within one month of creation) AVF thrombosis but failed to show improvement in long-term AVF patency, decrease rate of thrombosis in mature fistulas or any benefit for AVGs (19). In a randomized trial, fish oil was shown to decrease the occurrence of AVG thrombosis by 50% (20). The 2006 KDOQI guidelines do not address systemic anticoagulant use.
Pathophysiology of access thrombosis
Virshow’s triad of endothelial injury, stasis and hypercoagulability can be applied to dialysis access thrombosis (21).
Endothelial injury in dialysis access is multifaceted. One major component is abnormal vessel wall shear stress leading to endothelial dysfunction. Neointimal hyperplasia (discussed in detail below) leads to an altered composition of the endothelium with an abundance of extracellular matrix components on the luminal surface. Toxins from uremia also lead to endothelial dysfunction. Endothelial injury is also caused by dialysis needle punctures. Finally, in AVGs, no endothelium is present; there is an attendant 4× increase in thrombosis compared to AVFs (22,23). Endothelial injury from prior central venous catheter or transvenous pacemaker/defibrillator leads to central vein stenosis.
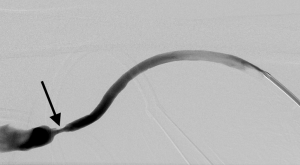
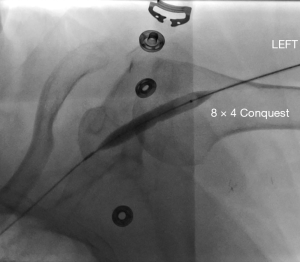
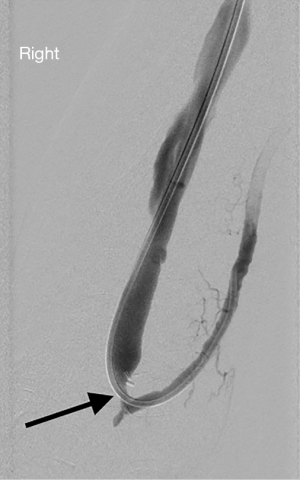
Stasis within a dialysis access can occur due to stenoses within the inflow, the conduit or the outflow. Poor inflow may be due to low cardiac output, systemic hypotension/hypovolemia or stenosis within the feeding artery. Within the conduit, sites of stenosis occur in relatively predictable locations. For AVGs, stenosis occurs just central to the graft-to-vein anastomosis in more than 85% of cases (24) (Figure 1). For AVFs, it depends on the type of fistula. For example, in a dysfunctional upper arm brachiocephalic fistula, the cephalic arch is stenosed in up to 77% of cases (Figure 2). In radiocephalic fistulas, the site of stenosis is the juxta-anastomotic segment in about 60% of cases (Figure 3) (25,26). These stenoses occur via a complex interplay of molecular and hemodynamic factors and have been studied most thoroughly at the graft-to-vein anastomosis. Cytokines such as hypoxia inducible factor-1α (HIF-1α), vascular endothelial growth factor-A (VEGF-A), matrix metalloproteinases (MMPs) and platelet-derived growth factor (PDGF) are produced by when the endothelium encounters turbulent flow, abnormal wall shear stress, vessel hypoxia and trauma. These cytokines induce the migration of fibroblasts and smooth muscles from the adventitia and media to the intima. There, they proliferate, causing stenosis (27). The hormone erythropoietin (EPO), which is often given to dialysis patients, induces proliferation of endothelial progenitor cells, leading to stenosis (28). Prolonged and/or overzealous compression after needle removal may also cause stasis and thrombosis.
Until recently, hypercoagulability has been underappreciated in its role in access thrombosis. While uremia is associated with impaired platelet function, dialysis patients are in an overall prothrombotic state (29). ESRD is a chronic inflammatory state that can be objectively shown by elevated C-reactive protein (CRP) levels. Elevated CRP has been shown to be a risk factor for access thrombosis (30). Other acquired prothrombotic risk factors seen in ESRD patients include hyperhomocysteinemia, low serum albumin and elevated lipoprotein levels (31). As blood circulates through the dialysis machine, it is exposed to an artificial membrane, which activates the clotting cascade and platelets (32-34). While these effects are counterbalanced by heparinization of the access circuit, an overall pro-thrombotic state remains. Inherited thrombophilias also play a role in access thrombosis. One study reported that 55% patients with access thrombosis had at least one thrombophilic condition (35). Two of the most commonly seen inherited thrombophilias are factor V Leiden and Prothrombin G20210A (36). In patients with recurrent access thrombosis, especially without identifiable stenosis, thrombophilia should be considered as a potential cause (37).
Manifestations of access thrombosis
AVG thrombosis extends the entire length of the graft, from the arterial to the venous anastomosis. The composition of the thrombus is predictable. At the arterial anastomosis, there is a ‘white’ clot, which is platelet rich. This platelet plug is resistant to tissue plasminogen activator (TPA) (38). Central to this is the ‘red clot’, which is fibrin rich and amenable to lysis with TPA. For AVFs the pattern of thrombosis is less predictable; it may involve a short segment or may extend to the level of the central veins.
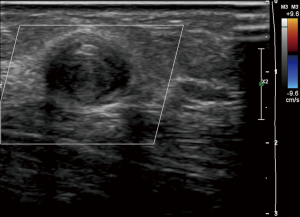
Access thrombosis is easily diagnosed when one encounters no thrill, bruit or pulse by physical exam. By ultrasound, there will be an absence of flow on pulse-wave and color Doppler. On gray scale ultrasound, non-compressible intraluminal echogenic material is seen (Figure 4). In thrombosed AVFs, inflammatory thrombophlebitis, which manifests as localized redness, pain and tenderness, may occur. Inflammatory thrombophlebitis from thrombosis does not occur in AVGs; if redness and pain are encountered in a thrombosed AVG, superimposed access infection is likely (39).
Contraindications to percutaneous AV access declotting
To avoid symptomatic pulmonary emboli, declotting should not be performed in patients with poor pulmonary or cardiac reserve (40). Heart disease is prevalent in the ESRD population. The cause of cardiac dysfunction in these patients is multifactorial. First, the two most common causes of ESRD (diabetes and hypertension) are also the two of the most important risk factors for developing heart disease. Right-sided heart failure is particularly prevalent in hemodialysis patients. Pulmonary hypertension and right ventricular dysfunction is estimated to occur about 1/3rd of ESRD patients (34,41,42). Anemia and chronic volume overload leads to pulmonary volume overload. High volume AV accesses (when Qa is >2 L/minute or >40% of the total cardiac output) play a causative role in the development of pulmonary hypertension. Pulmonary hypertension is seen at a significantly higher rate of patients dialyzed through AVFs or AVGs compared to those undergoing peritoneal dialysis or catheter based hemodialysis (43,44). Impaired right ventricular dysfunction can be assessed by evaluating for tricuspid annular plane excursion (TAPSE) (45).
Percutaneous declotting should be avoided in the setting of clotted mega-fistula as the large volume clot can cause symptomatic PE even in patients with normal heart and lung function. Percutaneous access declotting should not be performed in patients with a known right-to-left shunt as clot can go into the central arterial circulation, potentially causing stroke or other complications (46,47).
In cases of poor cardio-pulmonary reserve, right to left shunt or mega-fistula, surgical thrombectomy should be considered as thrombus is externally removed from the access. This is done by first making a 2 cm near the arterial anastomosis. The platelet plug is removed using an embolectomy catheter and occasionally using forceps. The downstream clot is manually milking thrombus through this incision (48).
In patients with AV access infection, percutaneous declot can lead to the precipitation of sepsis and the development of septic pulmonary emboli. In a patient with hemodynamic instability, hyperkalemia or severe coagulopathy, declot should be performed only after the patient is stablized. In a recently created AV access, percutaneous declotting should not be pursued for two reasons. First, the underlying problem likely needs to be fixed surgically. Second, fresh anastomotic suture lines may be disrupted (Figure 5). While individual practitioners vary, we suggest avoiding a percutaneous declot in an AV access which is less than 4–6 weeks old (2).
Procedural approach to percutaneous AV access declotting
Various techniques are described. Popular methods include lyse and wait (49), thromboaspiration (50), pulse spray aided pharmacomechanical thrombolysis (3), open surgical thrombectomy (51) and use of mechanical thrombectomy device such as the Arrow-Trerotola device (Teleflex; Wayne, PA, USA) (52).
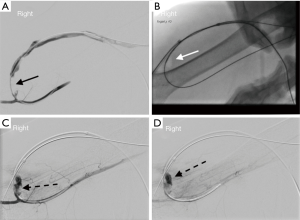
Pulse spray aided pharmacomechanical thrombolysis (Figure 6)
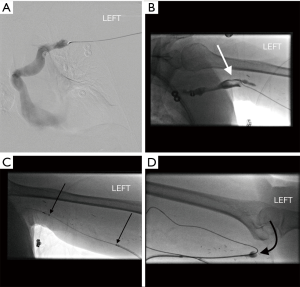
After one identifies the location arterial anastomosis and direction of flow within the access, the access is punctured just central to the arterial anastomosis in the direction of flow (antegrade direction) and a sheath is placed. Another sheath, at least 10 cm downstream from this antegrade sheath is placed facing the opposite direction (retrograde sheath). A wire and catheter are then advanced into the central veins and a venogram is performed. Any significant central vein stenosis is treated at this point. Then, the central most extension of clot is determined by performing a pullback injection. After this is done, an infusion catheter is placed through the antegrade sheath. Starting with the most central clot, TPA is sprayed into the clot with small (0.2 cc) forceful aliquots. Overlapping TPA injection is done as the catheter is intermittently retracted to the antegrade sheath. TPA is then pulse-sprayed into the clotted segment from the central most clot to the tip of the antegrade sheath. Balloon maceration of the clot is then performed, starting at the central end of the clot. After the outflow is cleared of clot, attention is turned towards the arterial plug. As mentioned above, the platelet rich arterial plug is not amenable to TPA lysis; mechanical thrombectomy is necessary. Through the retrograde sheath, a wire and catheter are gently advanced beyond the arterial anastomosis into the feeding artery. Then, the catheter is exchanged for a deflated Fogarty® embolectomy catheter (Edwards Lifesciences Irvine, CA, USA). After this catheter has been placed in the feeding artery, the balloon is inflated and pulled back, across the anastomosis and into the access pulling the plug into the access and centrally. The access should now be patent, which can be confirmed by physical examination, ultrasound, opening the side-arms of the sheaths or a small volume contrast injection. Once patency is confirmed, angiograms from the inflow to the right atrium should be performed and any inciting stenosis should be treated. Hemostasis can be achieved at the sheath sites with light manual pressure or with the application of purse-string sutures.
Mechanical thrombectomy with the Arrow-Trerotola device
Mechanical devices have been developed for or adapted to perform percutaneous declot. These devices include the Arrow-Trerotola, the AngioJetTM Peripheral Thrombectomy System (Boston Scientific; Marlborough, MA, USA) and the Argon Cleaner XTTM (Argon Medical, Plano, TX, USA). The Arrow-Trerotola device contains a rotating expandable nitinol basket that macerates thrombus as it spins at 3,000 revolutions per minute (RPMs). After placing two sheaths in the standard positions, central and pull back venograms are done and systemic heparinization is given. The compressed basket is advanced to the central most extension of clot, opened and turned on to macerate the clot while slowly pulling the device to the tip of the antegrade sheath. Once the basket reaches the tip of this sheath, the basket is collapsed and the device removed. Clot is aspirated through the sheath and the basket is cleaned. Then, the compressed basked is again advanced through this antegrade sheath and the process is repeated a few more times. A gentle angiogram can now be performed to evaluate for significant residual thrombus, which should be treated before proceeding. Once it appears that the outflow is clear of significant clot attention is turned to the inflow. The arterial plug can then be cleared using the Fogarty balloon described above or by using the basket of the Trerotola device. To use the Trerotola device, the compressed basket is advanced into the feeding artery. The basket is then opened but rotation not activated. The basket is gradually pulled back until it deforms, indicating that it is at the arterial anastomosis. At this location, the basket rotation turned on and the device slowly pulled back to the tip of the retrograde sheath. This is repeated a few times until the inflow has been cleared. A newer mechanical device by Argon medical called the CLEANER-XT™ uses a wire that rotates in a sinusoidal pattern at 4,000 RPMs. The amplitude of the sinusoidal wire matches the vessel diameter and creates a fluid vortex that macerates thrombus.
The lyse-and-wait technique
The lyse-and-wait technique utilizes the injection of TPA into the thrombosed access 30 to 120 minutes before the patient is brought to the room with a potential advantage being faster “in-room” procedure time (5). In the pre-procedure area, the access just downstream from the arterial anastomosis is prepped and draped. Antegrade puncture is done. While compressing the inflow and outflow of the access, 4 mg of TPA is injected. The catheter is secured in place and the TPA left to dwell until the procedure room is available. Subsequent initial angiogram may reveal minimal residual clot within the access outflow. The remaining steps are similar to steps described for pulse spray aided pharmacomechanical thrombolysis above.
Complications of declotting
Significant complications of percutaneous declotting, such as symptomatic pulmonary embolism or anastomotic disruption can be avoided with proper patient selection. Meticulous procedural technique can help minimize the risk of one of the more dreaded complications of declot, embolization of the platelet plug into the artery. Manipulation at the arterial anastomosis with wires, balloon and catheters should be minimized. Additionally, the operator should avoid pressurizing the access (e.g., doing angiogram within a clotted access) as the pressure could push the arterial plug into the feeding artery. The portion of the clot that embolizes into the distal artery is the platelet rich arterial plug. Therefore, mechanical removal via back-bleeding or endovascular balloon embolectomy, thromboaspiration or surgical thrombectomy rather than TPA lysis is necessary to remove this clot (2).
Outcomes
Technically successful declotting of an acutely thrombosed AV access can be achieved in >90% of cases. The rate of success is higher for AVGs than AVFs (53). While early patency rate is high, primary patency at one year is a dismal 10–20% (54).
Conclusions
HD access thrombosis leads to missed dialysis sessions, inpatient admissions, the occasional need for temporary dialysis catheter placement and is the leading cause of permanent access loss. Access thrombosis occurs in AVGs and AVFs approximately 0.5–2.0 and 0.1 to 0.5 times per year respectively. Pre-procedure evaluation should evaluate patients for contraindications to percutaneous declotting such as known right to left shunts, access infection and poor pulmonary reserve. Many different approaches to percutaneous declotting are used. Avoiding unnecessary manipulation near the arterial anastomosis can help decrease the chances of arterial embolization.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2016 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2017;69:A7-8. [Crossref] [PubMed]
- Quencer KB, Friedman T. Declotting the Thrombosed Access. Tech Vasc Interv Radiol 2017;20:38-47. [Crossref] [PubMed]
- Roberts AC, Valji K, Bookstein JJ, et al. Pulse-spray pharmacomechanical thrombolysis for treatment of thrombosed dialysis access grafts. Am J Surg 1993;166:221-5; discussion 225-6. [Crossref] [PubMed]
- Trerotola SO, Vesely TM, Lund GB, et al. Treatment of thrombosed hemodialysis access grafts: Arrow-Trerotola percutaneous thrombolytic device versus pulse-spray thrombolysis. Arrow-Trerotola Percutaneous Thrombolytic Device Clinical Trial. Radiology 1998;206:403-14. [Crossref] [PubMed]
- Cynamon J, Lakritz PS, Wahl SI, et al. Hemodialysis graft declotting: description of the "lyse and wait" technique. J Vasc Interv Radiol 1997;8:825-9. [Crossref] [PubMed]
- Koirala N, Anvari E, McLennan G. Monitoring and Surveillance of Hemodialysis Access. Semin Intervent Radiol 2016;33:25-30. [Crossref] [PubMed]
- Sirken GR, Shah C, Raja R. Slow-flow venous pressure for detection of arteriovenous graft malfunction. Kidney Int 2003;63:1894-8. [Crossref] [PubMed]
- Tessitore N, Bedogna V, Poli A, et al. Adding access blood flow surveillance to clinical monitoring reduces thrombosis rates and costs, and improves fistula patency in the short term: a controlled cohort study. Nephrol Dial Transplant 2008;23:3578-84. [Crossref] [PubMed]
- Moist LM, Churchill DN, House AA, et al. Regular monitoring of access flow compared with monitoring of venous pressure fails to improve graft survival. J Am Soc Nephrol 2003;14:2645-53. [Crossref] [PubMed]
- Salman L, Beathard G. Interventional nephrology: Physical examination as a tool for surveillance for the hemodialysis arteriovenous access. Clin J Am Soc Nephrol 2013;8:1220-7. [Crossref] [PubMed]
- Held PJ, Port FK, Wolfe RA, et al. The dose of hemodialysis and patient mortality. Kidney Int 1996;50:550-6. [Crossref] [PubMed]
- Daugirdas JT. Kt/V (and especially its modifications) remains a useful measure of hemodialysis dose. Kidney Int 2015;88:466-73. [Crossref] [PubMed]
- Quencer KB, Kidd J, Kinney T. Preprocedure Evaluation of a Dysfunctional Dialysis Access. Tech Vasc Interv Radiol 2017;20:20-30. [Crossref] [PubMed]
- Sands JJ. Vascular access monitoring improves outcomes. Blood Purif 2005;23:45-9. [Crossref] [PubMed]
- van Hinsbergh VW. Endothelium--role in regulation of coagulation and inflammation. Semin Immunopathol 2012;34:93-106. [Crossref] [PubMed]
- Neyra NR, Ikizler TA, May RE, et al. Change in access blood flow over time predicts vascular access thrombosis. Kidney Int 1998;54:1714-9. [Crossref] [PubMed]
- Vascular Access Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis 2006;48 Suppl 1:S248-73. [Crossref] [PubMed]
- Bandyk DF. Interpretation of duplex ultrasound dialysis access testing. Semin Vasc Surg 2013;26:120-6. [Crossref] [PubMed]
- Palmer SC, Di Micco L, Razavian M, et al. Antiplatelet therapy to prevent hemodialysis vascular access failure: systematic review and meta-analysis. Am J Kidney Dis 2013;61:112-22. [Crossref] [PubMed]
- Lok CE, Moist L, Hemmelgarn BR, et al. Effect of fish oil supplementation on graft patency and cardiovascular events among patients with new synthetic arteriovenous hemodialysis grafts: a randomized controlled trial. JAMA 2012;307:1809-16. [Crossref] [PubMed]
- Mammen EF. Pathogenesis of venous thrombosis. Chest 1992;102:640S-4S. [Crossref] [PubMed]
- Gibson KD, Gillen DL, Caps MT, et al. Vascular access survival and incidence of revisions: a comparison of prosthetic grafts, simple autogenous fistulas, and venous transposition fistulas from the United States Renal Data System Dialysis Morbidity and Mortality Study. J Vasc Surg 2001;34:694-700. [Crossref] [PubMed]
- Young EW, Dykstra DM, Goodkin DA, et al. Hemodialysis vascular access preferences and outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Int 2002;61:2266-71. [Crossref] [PubMed]
- Kanterman RY, Vesely TM, Pilgram TK, et al. Dialysis access grafts: anatomic location of venous stenosis and results of angioplasty. Radiology 1995;195:135-9. [Crossref] [PubMed]
- Sivanesan S, How TV, Bakran A. Sites of stenosis in AV fistulae for haemodialysis access. Nephrol Dial Transplant 1999;14:118-20. [Crossref] [PubMed]
- Hammes M, Funaki B, Coe FL. Cephalic arch stenosis in patients with fistula access for hemodialysis: relationship to diabetes and thrombosis. Hemodial Int 2008;12:85-9. [Crossref] [PubMed]
- Misra S, Fu AA, Rajan DK, et al. Expression of hypoxia inducible factor-1 alpha, macrophage migration inhibition factor, matrix metalloproteinase-2 and -9, and their inhibitors in hemodialysis grafts and arteriovenous fistulas. J Vasc Interv Radiol 2008;19:252-9. [Crossref] [PubMed]
- Reddy MK, Vasir JK, Hegde GV, et al. Erythropoietin induces excessive neointima formation: a study in a rat carotid artery model of vascular injury. J Cardiovasc Pharmacol Ther 2007;12:237-47. [Crossref] [PubMed]
- Weigert AL, Schafer AI. Uremic bleeding: pathogenesis and therapy. Am J Med Sci 1998;316:94-104. [PubMed]
- Chou CY, Kuo HL, Yung YF, et al. C-reactive protein predicts vascular access thrombosis in hemodialysis patients. Blood Purif 2006;24:342-6. [Crossref] [PubMed]
- Montagnana M, Meschi T, Borghi L, et al. Thrombosis and occlusion of vascular access in hemodialyzed patients. Semin Thromb Hemost 2011;37:946-54. [Crossref] [PubMed]
- Ando M, Iwata A, Ozeki Y, et al. Circulating platelet-derived microparticles with procoagulant activity may be a potential cause of thrombosis in uremic patients. Kidney Int 2002;62:1757-63. [Crossref] [PubMed]
- Cianciolo G, Stefoni S, Donati G, et al. Intra- and post-dialytic platelet activation and PDGF-AB release: cellulose diacetate vs polysulfone membranes. Nephrol Dial Transplant 2001;16:1222-9. [Crossref] [PubMed]
- Lucchi L, Ligabue G, Marietta M, et al. Activation of coagulation during hemodialysis: effect of blood lines alone and whole extracorporeal circuit. Artif Organs 2006;30:106-10. [Crossref] [PubMed]
- Knoll GA, Wells PS, Young D, et al. Thrombophilia and the risk for hemodialysis vascular access thrombosis. J Am Soc Nephrol 2005;16:1108-14. [Crossref] [PubMed]
- Atac B, Yakupoglu U, Ozbek N, et al. Role of genetic mutations in vascular access thrombosis among hemodialysis patients waiting for renal transplantation. Transplant Proc 2002;34:2030-2. [Crossref] [PubMed]
- Eleftheriadis T, Antoniadi G, Akritidou A, et al. A case report of recurrent vascular access thrombosis in a hemodialysis patient reveals combined acquired and inherited thrombophilia. Ther Apher Dial 2008;12:190-2. [Crossref] [PubMed]
- Sharafuddin MJ, Titus JL, Gu X, et al. Dialysis grafts arterial plug: retrieval using the tulip sheath device in vitro. Cardiovasc Intervent Radiol 1997;20:154-8. [Crossref] [PubMed]
- Ryan SV, Calligaro KD, Scharff J, et al. Management of infected prosthetic dialysis arteriovenous grafts. J Vasc Surg 2004;39:73-8. [Crossref] [PubMed]
- Sadjadi SA, Sharif-Hassanabadi M. Fatal pulmonary embolism after hemodialysis vascular access declotting. Am J Case Rep 2014;15:172-5. [Crossref] [PubMed]
- Yigla M, Banderski R, Azzam ZS, et al. Arterio-venous access in end-stage renal disease patients and pulmonary hypertension. Ther Adv Respir Dis 2008;2:49-53. [Crossref] [PubMed]
- Yigla M, Nakhoul F, Sabag A, et al. Pulmonary hypertension in patients with end-stage renal disease. Chest 2003;123:1577-82. [Crossref] [PubMed]
- Paneni F, Gregori M, Ciavarella GM, et al. Right ventricular dysfunction in patients with end-stage renal disease. Am J Nephrol 2010;32:432-8. [Crossref] [PubMed]
- Di Lullo L, Floccari F, Polito P. Right ventricular diastolic function in dialysis patients could be affected by vascular access. Nephron Clin Pract 2011;118:c257-61. [Crossref] [PubMed]
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685-713. [Crossref] [PubMed]
- Owens CA, Yaghmai B, Aletich V, et al. Fatal paradoxic embolism during percutaneous thrombolysis of a hemodialysis graft. AJR Am J Roentgenol 1998;170:742-4. [Crossref] [PubMed]
- Briefel GR, Regan F, Petronis JD. Cerebral embolism after mechanical thrombolysis of a clotted hemodialysis access. Am J Kidney Dis 1999;34:341-3. [Crossref] [PubMed]
- Cull DL, Washer JD, Carsten CG, et al. Description and outcomes of a simple surgical technique to treat thrombosed autogenous accesses. J Vasc Surg 2012;56:861-5. [Crossref] [PubMed]
- Cynamon J, Pierpont CE. Thrombolysis for the treatment of thrombosed hemodialysis access grafts. Rev Cardiovasc Med 2002;3 Suppl 2:S84-91. [PubMed]
- Turmel-Rodrigues L, Sapoval M, Pengloan J, et al. Manual thromboaspiration and dilation of thrombosed dialysis access: mid-term results of a simple concept. J Vasc Interv Radiol 1997;8:813-24. [Crossref] [PubMed]
- Ponikvar R. Surgical salvage of thrombosed arteriovenous fistulas and grafts. Ther Apher Dial 2005;9:245-9. [Crossref] [PubMed]
- Lazzaro CR, Trerotola SO, Shah H, et al. Modified use of the arrow-trerotola percutaneous thrombolytic device for the treatment of thrombosed hemodialysis access grafts. J Vasc Interv Radiol 1999;10:1025-31. [Crossref] [PubMed]
- Kakkos SK, Haddad GK, Haddad J, et al. Percutaneous rheolytic thrombectomy for thrombosed autogenous fistulae and prosthetic arteriovenous grafts: outcome after aggressive surveillance and endovascular management. J Endovasc Ther 2008;15:91-102. [Crossref] [PubMed]
- Schon D, Mishler R. Pharmacomechanical thrombolysis of natural vein fistulas: reduced dose of TPA and long-term follow-up. Semin Dial 2003;16:272-5. [Crossref] [PubMed]


