Is universal grading of diastolic function by echocardiography feasible?
Left ventricular (LV) diastolic function: basic physiology
Diastolic function reflects the ability of the heart to change its shape in order to receive blood from the venous system. This property of the heart is essential as it allows myocyte stretch and thus regulation of systolic output through activation of Frank Starling (heterometric) mechanism without necessitating a change in contractility (1).
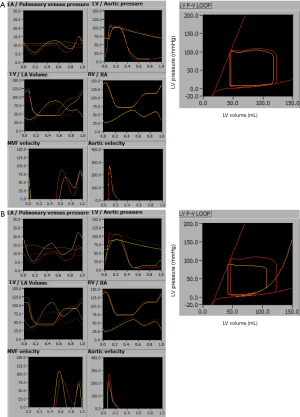
Quantitation of diastolic function centers on the assessment of active (energy dependent) and passive (energy independent) ventricular properties, and involves measurement of ventricular relaxation (active property), and chamber and myocardial stiffness (passive properties). Myocardial relaxation reflects the exponential drop (or, in physics terms, rate of change) of LV pressure decrease once LV ejection ends, and is measured by time constant of isovolumic pressure decay (tau) (2). Pressure decrease, and thus relaxation, is essential, as otherwise no inflow of blood into the ventricle would occur. Myocardial relaxation is strictly a property of myocytes, is influenced by myofibrillar structure and calcium metabolism, and is relevant only during initial part of the diastole (to be precise, during time interval that is equal to 3.5 times tau, which is approximately 170 ms) (3). Myocardial stiffness in turn reflects the elasticity of myocardial tissue and is quantified by the way a relaxed myocardium stretches under stress (i.e., how it confirms to the law of Laplace) (2). In contrast to relaxation, it depends on both intra- (4) and extracellular matrix structure [i.e., collagen content (5)], is not linked to calcium metabolism, and affects second half of the diastolic period. Chamber stiffness reflects elasticity (or compliance) of the ventricle and is quantified by analyzing the way ventricular pressure increases with increase in ventricular volume (2). The simplest (and thus not completely accurate) measure of chamber stiffness is operative chamber stiffness, which represents stroke volume divided by difference between minimum and maximum ventricular diastolic pressure (6). Chamber stiffness is dictated by myocardial stiffness and chamber shape. The easiest way to understand this relationship between myocardial and chamber stiffness is the analogy between bricks and the house: a “solid” (stiff) house (ventricle) can be made from either solid (‘stiff’) bricks (i.e., myocardial fibrosis) or from ordinary bricks with thick walls (i.e., hypertrophy, concentric remodeling) (2). Figure 1 shows computer simulation of left atrial and ventricular pressures and volumes, as well as corresponding transmitral flows, in the normal heart, in heart with slow relaxation (Figure 1A), and in a heart with increased chamber stiffness (Figure 1B).
LV diastolic dysfunction: definition
In order to quantitate a particular feature, one has to have a simple, universal, and unambiguous definition. This type of definition of diastolic dysfunction is lacking, due to complex underlying physiology (7). The best way to define it for the purpose of this manuscript would be the propensity of the left ventricle to develop increased filling pressure [with filling pressure defined as LV end-diastolic pressure (LVEDP)]. Distinction to be made is that diastolic dysfunction is not identical to increased LV filling pressure—one can have LV diastolic dysfunction even if LVEDP is normal (e.g., a well-diuresed patient with ischemic cardiomyopathy). On the other hand, one can have elevated LVEDP despite having normal diastolic function (example would be normal subject after large saline infusion). One should also not confuse LV filling pressure with left atrial or pulmonary capillary wedge pressure as both of them can be elevated in the presence of mitral valve disease, while pulmonary capillary wedge pressure may in addition be elevated in the setting of pulmonary vein stenosis. Finally, one should make a clear distinction between LV diastolic dysfunction and dysfunction due to factors that are extrinsic to the LV myocardium, such as primary pulmonary hypertension, external compression, or pericardial constriction/effusion.
What are potential origins of diastolic dysfunction?
As already said, diastolic dysfunction is a propensity to develop increased LVEDP. However LVEDP can increase by mechanisms that are extrinsic or intrinsic to the heart or left ventricle. Acute volume overload (e.g., seen with water intoxication) would be an example of extrinsic mechanism. Intrinsic mechanisms would involve a change of myocardial properties or in LV shape. Myocardial properties can change in two ways: one is by prolonging (delaying) the relaxation, the other by the increase in LV myocardial stiffness. It is important to understand that, while worsening of these two parameters often occurs concomitantly, it is not always the case, and pathophysiology of these two processes is completely different. Relaxation is energy dependent process that depends on calcium sequestration back into sarcoplasmic reticulum. Passive properties rely on the structure of extracellular matrix (amount of fibrosis) and intramyocyte skeleton (titin expression) (8). While processes such as ischemic heart disease can influence both relaxation (through ischemia) and passive elastic properties (through replacement fibrosis post ischemic damage), these pathophysiologic processes are different, and do not necessarily coexist. This is important to understand, as often described sequence of progressive diastolic dysfunction going through stages of impaired relaxation, pseudonormal filling and restrictive physiology is not necessarily seen in every patient.
Another, very important mechanism, is related to change in chamber properties, where LV concentric remodeling [e.g., seen with bed rest (9) or with aging (10)], resulting in increased relative LV wall thickness despite the change in mass, leads to increased chamber stiffness without actual change in myocardial properties.
It is important to mention that neither change in myocardial or chamber properties necessarily leads to increase in LVEDP. For this to happen, additional factors are often needed-this would be increased heart rate in the setting of delayed relaxation, or a volume overload of any etiology in the presence of increased myocardial stiffness.
Difference between diastolic function parameters, diastolic parameters and diastolic function-influenced parameters
While parameters of diastolic function are well defined, their accurate measurement almost always necessitates some invasive procedure. Because of this, multiple noninvasive parameters were proposed as surrogates. Most of these noninvasive parameters rely on measurement of some cardiac feature performed during the diastolic phase of the cardiac cycle, making them a diastolic parameter. However, the fact that some parameter is derived from the measurement that is performed during diastole does not necessarily mean that it is solely determined by diastolic function. The example of it would be early diastolic velocity of the mitral annulus, which is influenced by multiple non-diastolic factors, such as body size (11) and long-axis systolic function (12).
In addition, there are multiple diastolic function-influenced parameters that are not measured in diastole and not solely determined by diastolic dysfunction. The example of these parameters would be right ventricular systolic pressure (which can be influenced by pulmonary stenosis or pulmonary hypertension) or left atrial size [which is influenced by the history of mitral regurgitation, stenosis, or atrial fibrillation (AF)]. In other words, understanding diastolic function in clinical setting can often be difficult if one does not take account of confounding factors.
Understanding echocardiographic diastolic parameters
The number of diastolic parameters that could be obtained by echocardiography is staggering. Table S1 lists some of them, grouped in the categories of simple or composite (i.e., using some type of calculation), along with their potential validation as markers of LV stiffness, relaxation or filling pressure. While the number of parameters may appear discouraging, in everyday assessment only a limited few are used, with E and A wave velocities of mitral inflow and E' velocity of mitral annulus, along with parameters derived from them, most frequently reported.

Full table
However, even if we limit the number of diastolic parameters, several factors may hinder their interpretation. The first issue is that every echo parameter is affected by measurement error. The second is that all echo parameters are influenced by more than one LV function parameter. The third is that echo parameters almost never show direct mathematical relationship with their purported major physiologic parameter. Case in hand is E wave velocity. E wave velocity measurement carries significant error, as was very recently shown by some of the leaders in the field of echocardiography (13). In addition, E wave velocity is traditionally assumed to be determined by LVEDP and LV relaxation. But on top of this E wave is also very sensitive to mitral valve narrowing (14) mitral regurgitation (15), and hyperdynamic circulation in the setting of anemia or septic shock. Importantly, there is no mathematical equation that links E wave velocity to LV filling pressure in a way that transaortic valve velocities are linked to aortic valve gradients by Bernoulli equation.
Using composite parameters
We use the term composite to describe parameters that are obtained by combination of two or more directly measured parameters. The two most frequently used composite parameters, E/A and E/E', represent the mainstay of echocardiographic assessment of diastolic function. The rationale behind their use is that combination of parameters can better reflect diastolic function than use of individual one. In the case of E/E', it is assumed that E’ is more sensitive to relaxation than on preload, and thus by using it to divide E wave velocity, it eliminates impact of relaxation on E wave thus making it sensitive only to LV preload. The problem with this approach is that combining two parameters increases measurement error by default. In addition, E/E' and E/A ratios were proposed based on empirical observations. Thus, there is no analytic proof that E/E' ratio should reflect LV filling pressure or stiffness, nor is there validation of this ratio against any other combination of the two constitutive parameters, E and E'.
Grading diastolic function by echocardiography: a brief history
The original observations of Drs. Appleton, Hatle and Popp (16) introduced the concept of attributing stages (i.e., levels, grades) of diastolic dysfunction based on the shape of mitral inflow. It was noted that patients with poor LV relaxation have decreased E/A ratio, with it becoming “pseudonormal” or even higher than normal once elevation of filling pressures occur. Based on this the authors proposed grading of mitral inflow pattern as normal; impaired relaxation; pseudonormal; and restrictive (meaning there was restriction to inflow from pulmonary veins). The issue that was immediately apparent was that it was not possible to differentiate normal from pseudonormal pattern just based on E/A ratio-which invited other echocardiographic parameters into play. Introduction of these, additive, parameters led to efforts to lump characteristic patterns of several parameters together, in order to classify patients into appropriate subsets. An example of this process is illustrated by ad hoc classification proposed by Redfield et al. (17) where six non-hierarchical parameters (Table 1) are used to classify patients into five categories (with distinction between restrictive “reversible” and “irreversible” grades). Interestingly, from the standpoint of validation of diastolic function grading scheme, this study was negative, as it did not show that diastolic dysfunction severity influenced patients’ outcomes. The main practical issue with this and similar classification systems is that, as by nature individual echocardiographic measurements vary because of measurement error and beat-to-beat variability, their combination will vary even more, creating often difficult-to-interpret groupings of parameters, some of them pointing to the one, and some to the other degree of dysfunction. There are also other issues, and the authors’ subsequent manuscript, based on a healthy subset derived from the same population, offers a thorough review of additional difficulties associated with this type of classification (18). Among other findings, the authors show that measuring duration of atrial flow reversal on pulmonary vein flow is not always possible; that response of mitral inflow to Valsalva maneuver in healthy subjects may vary from none to more than 50%; and most importantly, that almost all parameters vary dramatically with age.

Full table
To improve on this and make grading diastolic function uniform, American Society of Echocardiography and European Association of Echocardiography (ASE/EAE) proposed a classification that relies on a more complex scheme with a total of 8 parameters organized in a single, two-step, decision tree (Table 1) (19). However, a final step still involved decision making based on five non-hierarchical parameters—in other words, grader had to rely on his subjective decision whether to be guided by one versus the other parameter. This classification scheme also included LA volume index (LAVi) as one of the markers of diastolic function, although LAVi is neither a parameter of diastolic function nor a diastolic parameter (see above), as well as Ar-A and Valsalva maneuver, already shown to be unreliable.
Given these very obvious problems, it is not surprising that ASE and European Association of Cardiovascular Imaging (ASE/EACVI) recently proposed a new grading system, which, despite a claim of being only an update, represents a radical departure from the prior one (20). The major change was introduction of two, instead of a single decision tree. The first (“triage”) decision tree us used to divide patients into groups of normal, abnormal, or “indeterminate” diastolic function. However, not all patients should undergo this step. According to the actual text of ASE/EACVI Recommendations, patients with abnormal LV ejection fraction (LVEF) would be assumed to have abnormal diastolic function and should proceed directly to the second decision tree. This recommendation is confusing, as it is unclear why one should assume that, for an example, a 40 year old female breast cancer patient undergoing trastuzumab chemotherapy with LVEF of 53% (which is below lower limit of normal according to 2016 ASE/EACVI Recommendations) (21) should be automatically assumed to have LV dysfunction. What is even more confusing is that ASE provides a webinar which suggests that the first step should be skipped not only in the cases of decreased LVEF, but in all cases where clinical data are consistent with the presence of myocardial disease (22). If we follow this line of reasoning, any patient with structural heart disease could be excluded, including a patient with concentric remodeling, LV hypertrophy, mitral annular calcification, prior coronary artery intervention, mitral valve prolapse, etc. If one would hold to this suggestion, in a large tertiary clinical center the first step would have to be skipped in practically all patients, and therefore for the purpose of this review we can neglect its existence.
Which leaves us with the second (“classification”) decision tree that is used to classify patients into three diastolic dysfunction grades (normal, mild, severe) and “cannot determine” slot left for patients with incongruent data. The tree performs a two-step classification based on E/A ratio as a first step followed by additional reclassification in patients with what was formerly known as “pseudonormal” pattern. This second step relies on E/E', LAVi and tricuspid regurgitation (TR) velocities (a reflection of right ventricular systolic pressures, RVSP)—in other words, 2 out of 3 parameters are neither parameters of diastolic function nor diastolic parameters. There are multiple issues with this classification, the most glaring one being that classification is unclear in what is E/E' cutoff to determine “pseudonormal” pattern, as classification tree cites the value of 14, while the fourth table of the 2016 ASE/EACVI Recommendations manuscript cites E/E' the value of 10. Introduction of TR as a parameter is especially questionable, as it reflects the presence of pulmonary hypertension which can have multiple other causes, and in addition is not even a precise measure of it, especially if one deals with low risk patients (23). One can envisage multiple scenarios, such as a presence of mitral stenosis due to mitral annular calcifications resulting in enlarged LA and increased TR velocities that would lead to categorization of the patient as having diastolic dysfunction, with LV diastolic function being completely normal (and with LVEDP actually lower than what it should be). Even these issues pale compared to two major ones—that no specific effort is made to account for the changes occurring with aging, or for the presence of known pathology.
Is aging a pathologic process?
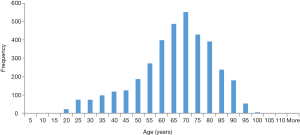
Somewhat paradoxically, this seemingly trivial question is a central issue in assessing diastolic function. Aging is associated with homeostasis, i.e., diminishing physiologic reserves available to preserve homeostasis. Aging does not affect resting left atrial pressure (24), but it leads to increased LV chamber stiffness and slowing of relaxation, which makes cardiovascular system more susceptible to stress induced by either disease or exercise. The result of these changes is that all echocardiographic parameters that are associated with relaxation worsen dramatically with aging (25). This decrease in relaxation does not start in what is considered a middle age—it starts with adolescence (26). Because of that, if average values of diastolic parameters measured during a second decade of life are used to define normal values, most healthy people 40–60 years old would be considered as having a diastolic dysfunction. Aging also leads to an increase in RVSP (27,28), so much so that even RVSP of 40 mmHg may be normal in elderly, especially if obese. While this is a phenomenon in healthy elderly, it increases even more in a concomitant presence of pulmonary disease, whose prevalence also increases with age. Figure 2 shows age-related normal values of, septal E', E/E', E/A and RVSP along with cutoff values proposed in 2016 Recommendations. As one can see, while the cutoff value of 14 for E/E' does exclude almost all normal values, this is not so for cutoff value of 10, or for septal E' and E/A ratio whose cutoff values of <7 cm/s and >0.8 equal average values past the age of 70 years. In sum, values of E' of <7 cm/s for the septum and 10 cm/s for the lateral wall, TR velocity of >2.8 cm/s, E/A ratio of <0.8, E/E' of >10 occur individually in up to 50% of patients older than 70 years, making this algorithm particularly overly sensitive. Given this, it would be almost impossible to classify a patient who is more than 70 years old as not having a diastolic dysfunction, which, if any mitral valve pathology such as mitral annular calcification is present (see below), would lead to E wave velocities of >50 cm/s, thus with patient being classified as having grade II diastolic dysfunction. These issues are relevant, as patients >70 years of age are much more likely to require echocardiogram and are “overrepresented” in the clinical setting. In fact, at Cleveland Clinic, most of the patients that required echocardiogram in year 2016 are 70 years of age or over (Figure 3).

Presence of known pathology
Echocardiogram represents by far the most frequently used method to assess the presence of structural heart disease. Interpretation of diastolic dysfunction thus always occurs in the setting of particular constellation of findings which point to a specific pathology. It is well known that a specific pathology is associated with specific pattern of changes in diastolic parameters-perhaps the best example being signature constellation of high E and E' velocities and large left atrium in constrictive pericarditis (29). However, other pathologies are also associated with a specific pattern in abnormalities of diastolic parameters. We below discuss some of them.
Absent or improperly timed left atrial contraction
We are both living in AF epidemic and have more options to keep patients out of it by direct cardioversion (DCV), pharmacologic or non-pharmacologic rhythm control. However, the successes in AF treatment are rarely curative, AF may recur, and residual changes, including LA increase and loss of atrial contraction, even if ameliorated, persists (Figure 4). In other words, once a patient has AF, it may be expected that he/she will have some form of sequel to atrial structure and function forever. While atrial contraction is part of a diastolic process, it really is not part of LV function. For the purpose of classification, loss or diminution of atrial contraction dramatically changes E/A ratio without any change in diastolic properties. Should one consider that this patient has severe (grade III) diastolic dysfunction despite the presence of NSR being considered a success after prior AF ablation?

Another special population is patients with pacemakers. Patient with VVI PPM pacemaker do not have atrial kick. But even more so, in patients with DDD pacemakers, E/A ratio can be manipulated, especially in the setting of cardiac resynchronization therapy (30). How should these patients be categorized? It does not help that right ventricular pacing leads to abnormal contraction pattern that in turn influences long axis function.
Mitral valve disease
It is not necessary to have severe mitral regurgitation or stenosis to impact transmitral velocities. Some mild to moderate mitral valve pathology is almost always present in older individuals. Appearance of mitral annular calcifications, often associated with decrease in mitral valve area, and mild to moderate early systolic mitral regurgitation is ubiquitous in the eighth and ninth decade of life. Both of these processes increase E wave velocities, which is reflective of higher flow across, and smaller area of the mitral valve rather than elevation of LVEDP. In addition, this may lead to increased LA and pulmonary vein pressure-even with normal or lower than normal LVEDP. According to the guidelines, these patients will be categorized as having diastolic dysfunction, and while it is true that they do have abnormality, it is not necessarily due to abnormality of the left ventricle.
There are multiple other conditions where applicability of any diastolic function grading system is dubious. In hypertrophic cardiomyopathy an E/A ratio consistent with “impaired relaxation” predominates even in symptomatic patients undergoing septal myectomy (31). Patients with constrictive pericarditis have increased E’ wave velocities; in fact, this is one of the parameters used to diagnose this condition (29). Presence of aortic regurgitation leads to increase in E/A ratio so much so that A wave can even be absent (32). Pulmonary hypertension of any kind (including the one cause by parenchymal pulmonary disease, autoimmune disease and others) leads not only to increase in TR velocities, but in changes LV systolic and diastolic parameters (33). Non-cardiac clinical conditions can influence diastolic function assessment. Anemia of any cause increases transmitral velocities (34,35). The same occurs in advanced liver disease, and in patients undergoing hemodialysis. In sum, it is almost impossible to have a hospitalized patient in whom classification, as above, can be applied without reservations.
Is diagnosis of diastolic dysfunction additive to already available knowledge?
Prior to being published, neither prior, nor the current guidelines were validated as a prognostic tools—they were proposed ad hoc. While individual elements of the algorithm may correlate with the event free survival (36), it is unknown whether current algorithm represents the best possible mixture of elements and cut off points. With a hindsight, one could envisage a study that used prior database to construct an empirically derived decision making tree [e.g., using CART analysis (37)], or develop a prognostic scoring system analogous to CHA2DS2-VASC score. However, this was not done with this classification, and thus question of its utility remains unanswered.
How can we apply recommendations in clinical practice?
Reporting diastolic function, according to current ASE recommendations (38), is an integral part of an echocardiographic report. The only appropriate way to report diastolic function is by following current recommendations. In other words, diastolic dysfunction grade determination is valid only if current guidelines, in some shape, are implemented. However, as we point out, guidelines are ambiguous and with cutoff points that may misclassify patients as both sicker and healthier than they are. Even more importantly, in the current era of greatly facilitated patient access to medical reports, it may misinform them. In other words, implementing diastolic function guidelines, as proposed, is impossible. Hopefully, next iteration of recommendations would overcome these issues.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Solaro RJ. Mechanisms of the Frank-Starling law of the heart: the beat goes on. Biophys J 2007;93:4095-6. [Crossref] [PubMed]
- Glantz SA, Parmley WW. Factors which affect the diastolic pressure-volume curve. Circ Res 1978;42:171-80. [Crossref] [PubMed]
- Weisfeldt ML, Frederiksen JW, Yin FC, et al. Evidence of incomplete left ventricular relaxation in the dog: prediction from the time constant for isovolumic pressure fall. J Clin Invest 1978;62:1296-302. [Crossref] [PubMed]
- LeWinter MM, Wu Y, Labeit S, et al. Cardiac titin: structure, functions and role in disease. Clin Chim Acta 2007;375:1-9. [Crossref] [PubMed]
- Hess OM, Ritter M, Schneider J, et al. Diastolic stiffness and myocardial structure in aortic valve disease before and after valve replacement. Circulation 1984;69:855-65. [Crossref] [PubMed]
- Little WC, Ohno M, Kitzman DW, et al. Determination of left ventricular chamber stiffness from the time for deceleration of early left ventricular filling. Circulation 1995;92:1933-9. [Crossref] [PubMed]
- Grossman W.. Diastolic dysfunction in congestive heart failure. N Engl J Med 1991;325:1557-64. [Crossref] [PubMed]
- Nagueh SF, Shah G, Wu Y, et al. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation 2004;110:155-62. [Crossref] [PubMed]
- Perhonen MA, Zuckerman JH, Levine BD. Deterioration of left ventricular chamber performance after bed rest: "cardiovascular deconditioning" or hypovolemia? Circulation 2001;103:1851-7. [Crossref] [PubMed]
- Arbab-Zadeh A, Dijk E, Prasad A, et al. Effect of aging and physical activity on left ventricular compliance. Circulation 2004;110:1799-805. [Crossref] [PubMed]
- Popovic ZB, Sun JP, Yamada H, et al. Differences in left ventricular long-axis function from mice to humans follow allometric scaling to ventricular size. J Physiol 2005;568:255-65. [Crossref] [PubMed]
- Popovic ZB, Desai MY, Buakhamsri A, et al. Predictors of mitral annulus early diastolic velocity: impact of long-axis function, ventricular filling pattern, and relaxation. Eur J Echocardiogr 2011;12:818-25. [PubMed]
- Farsalinos KE, Daraban AM, Unlu S, et al. Head-to-Head Comparison of Global Longitudinal Strain Measurements among Nine Different Vendors: The EACVI/ASE Inter-Vendor Comparison Study. J Am Soc Echocardiogr 2015;28:1171-81.e2. [Crossref] [PubMed]
- Thomas JD, Newell JB, Choong CY, et al. Physical and physiological determinants of transmitral velocity: numerical analysis. Am J Physiol 1991;260:H1718-31. [PubMed]
- Thomas JD, Zhou J, Greenberg N, et al. Physical and physiological determinants of pulmonary venous flow: numerical analysis. Am J Physiol 1997;272:H2453-65. [PubMed]
- Appleton CP, Hatle LK, Popp RL. Relation of transmitral flow velocity patterns to left ventricular diastolic function: new insights from a combined hemodynamic and Doppler echocardiographic study. J Am Coll Cardiol 1988;12:426-40. [Crossref] [PubMed]
- Redfield MM, Jacobsen SJ, Burnett JC Jr, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003;289:194-202. [Crossref] [PubMed]
- Munagala VK, Jacobsen SJ, Mahoney DW, et al. Association of newer diastolic function parameters with age in healthy subjects: a population-based study. J Am Soc Echocardiogr 2003;16:1049-56. [Crossref] [PubMed]
- Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009;22:107-33. [Crossref] [PubMed]
- Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277-314. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1-39.e14. [Crossref] [PubMed]
- Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277-314. [Crossref] [PubMed]
- Parent F, Bachir D, Inamo J, et al. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med 2011;365:44-53. [Crossref] [PubMed]
- Prasad A, Okazaki K, Arbab-Zadeh A, et al. Abnormalities of Doppler measures of diastolic function in the healthy elderly are not related to alterations of left atrial pressure. Circulation 2005;111:1499-503. [Crossref] [PubMed]
- Daimon M, Watanabe H, Abe Y, et al. Normal values of echocardiographic parameters in relation to age in a healthy Japanese population: the JAMP study. Circ J 2008;72:1859-66. [Crossref] [PubMed]
- Okura H, Takada Y, Yamabe A, et al. Age- and gender-specific changes in the left ventricular relaxation: a Doppler echocardiographic study in healthy individuals. Circ Cardiovasc Imaging 2009;2:41-6. [Crossref] [PubMed]
- McQuillan BM, Picard MH, Leavitt M, et al. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation 2001;104:2797-802. [Crossref] [PubMed]
- Lam CS, Borlaug BA, Kane GC, et al. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation 2009;119:2663-70. [Crossref] [PubMed]
- Kusunose K, Dahiya A, Popovic ZB, et al. Biventricular mechanics in constrictive pericarditis comparison with restrictive cardiomyopathy and impact of pericardiectomy. Circ Cardiovasc Imaging 2013;6:399-406. [Crossref] [PubMed]
- Gorcsan J 3rd, Abraham T, Agler DA, et al. Echocardiography for cardiac resynchronization therapy: recommendations for performance and reporting--a report from the American Society of Echocardiography Dyssynchrony Writing Group endorsed by the Heart Rhythm Society. J Am Soc Echocardiogr 2008;21:191-213. [Crossref] [PubMed]
- Desai MY, Bhonsale A, Smedira NG, et al. Predictors of long-term outcomes in symptomatic hypertrophic obstructive cardiomyopathy patients undergoing surgical relief of left ventricular outflow tract obstruction. Circulation 2013;128:209-16. [Crossref] [PubMed]
- Vilacosta I, San Roman JA, Castillo JA, et al. Retrograde atrial kick in acute aortic regurgitation. Study of mitral and pulmonary venous flow velocities by transthoracic and transesophageal echocardiography. Clin Cardiol 1997;20:35-40. [Crossref] [PubMed]
- Puwanant S, Park M, Popovic ZB, et al. Ventricular geometry, strain, and rotational mechanics in pulmonary hypertension. Circulation 2010;121:259-66. [Crossref] [PubMed]
- Bosi G, Crepaz R, Gamberini MR, et al. Left ventricular remodelling, and systolic and diastolic function in young adults with beta thalassaemia major: a Doppler echocardiographic assessment and correlation with haematological data. Heart 2003;89:762-6. [Crossref] [PubMed]
- Zilberman MV, Du W, Das S, et al. Evaluation of left ventricular diastolic function in pediatric sickle cell disease patients. Am J Hematol 2007;82:433-8. [Crossref] [PubMed]
- Liang HY, Cauduro SA, Pellikka PA, et al. Comparison of usefulness of echocardiographic Doppler variables to left ventricular end-diastolic pressure in predicting future heart failure events. Am J Cardiol 2006;97:866-71. [Crossref] [PubMed]
- Tsutsui RS, Borowski A, Tang WH, et al. Precision of echocardiographic estimates of right atrial pressure in patients with acute decompensated heart failure. J Am Soc Echocardiogr 2014;27:1072-8.e2. [Crossref] [PubMed]
- Picard MH, Adams D, Bierig SM, et al. American Society of Echocardiography recommendations for quality echocardiography laboratory operations. J Am Soc Echocardiogr 2011;24:1-10. [Crossref] [PubMed]


