Imaging work-up and endovascular treatment options for aorto-enteric fistula
Introduction
Aorto-enteric fistula (AEF) is a rare though life-threatening condition that is formed when the aorta erodes through the wall of an adjacent segment of the gastrointestinal (GI) tract, leading to sepsis or hemorrhage if untreated. The current management of AEFs remains controversial. AEFs are traditionally classified as either primary or secondary, with the majority attributed to the latter category (1). Primary AEFs can occur as a direct consequence of large untreated descending thoracic or abdominal aortic aneurysms (AAAs) with or without associated infection (mycotic aneurysm or aortitis), eroding through the bowel wall, or due to bulky para-aortic tumoral tissue invading through the aortic wall. Secondary AEFs (SAEFs) are more common than primary AEFs and generally develop as a result of long-term sequelae of either surgical or endovascular aortic reconstruction, with no significant difference in SAEF incidence between the two approaches (2). Most AEFs develop between the duodenum and the aorta; however, they can occur in almost any section of the GI tract (3).
Because AEFs can cause death by exsanguination if left untreated, it is crucial to recognize and treat AEFs as rapidly as possible (4,5). However, AEFs can be difficult to diagnose due to their rarity and the multitudinous causes of GI bleeding. Clinicians should be cognizant to include SAEF on the differential diagnosis of GI bleeding, especially if the patient has a history of AAA reconstruction.
SAEFs can be managed by pursuing either a surgical or an endovascular approach. Surgical techniques used to treat SAEF include aortic graft/stent excision, with either in situ aortic reconstruction or extra-anatomic bypass (1,6,7). Recent estimates of early post-operative mortality for these open surgical techniques vary widely, but remain high, in the range of 18–54% (8,9). As a result of these high mortality rates, endovascular interventions have grown in popularity to manage AEF patients.
The most commonly used endovascular SAEF management technique is stent-graft repair. Endovascular balloon occlusion or coil embolization can also be used to quickly stop hemorrhagic blood loss. Based on prior studies there appears to be a profound early survival benefit when endovascular management of SAEFs is performed compared with surgical management. Some of this benefit is lost when analyzing long-term outcome data, primarily due to the increased rates of recurrence and sepsis associated with some endovascular techniques. Despite this, long term survival rates still appear higher with endovascular management of SAEFs compared to open surgery (3).
Given the relatively small number of cases of SAEF treated with endovascular techniques, more evidence in a multicenter approach is required to conduct a thorough comparison and obtain reliable long-term outcome data. Furthermore, by adding more cases to the compendium of literature, it may eventually be possible to determine the contributing factors to recurrence and sepsis in SAEF patients managed with endovascular treatment. Gaining knowledge about these factors may allow for appropriate interventions to be made to greatly improve outcomes. Moreover, surgical and endovascular management of SAEF can be integrated in a complementary approach. There have been suggestions of using endovascular management as a bridge to open repair (10).
In addition to elaborating on the current literature with a focus on endovascular treatment of SAEF we present four representative cases of AEF with imaging findings. Three patients had aorto-esophageal fistula and one patient was found to have an aorto-jejunal fistula. AEFs in these locations are less common, so discussing these cases may assist in early differential consideration, diagnosis, and management of future AEFs.
Clinical presentation of AEF
The most common presentation of SAEF is GI bleeding (11-13). GI bleeding in SAEF can follow a distinct pattern in which patients encounter one or more smaller ‘herald bleeds’ before experiencing massive hemorrhage hours or months later (14,15). These massive hemorrhages may be life threatening. However, between 27% and 60% of SAEFs do not present with GI bleeding (1,6,8). The most common presentation for SAEF without GI bleeding is sepsis, which occurs in up to 80% of SAEF patients (some of these may have concomitant GI bleeding) (16,17).
The reason why some SAEFs present with GI bleeding and others do not is presumably related to the type of SAEF (18). Anastomotic fistulas in the postsurgical setting involve the graft-aortic suture line. The constant pulsation of the aorta likely causes the suture line to gradually erode through the bowel wall leading to massive GI hemorrhage. In contrast, paraprosthetic fistulas do not involve the suture line, but the body of the graft which erodes through the wall of the bowel, allowing bacterial translocation from bowel flora into the aorta, thereby enhancing susceptibility to sepsis (16,19,20).
Diagnostic work-up of AEF
Timely diagnosis of SAEF is critical and requires a high degree of suspicion when a patient presents with signs of GI hemorrhage or sepsis. While SAEFs are frequently diagnosed intraoperatively during laparotomy, and are occasionally detected pre-operatively with endoscopy, CT angiography is the diagnostic modality of choice (3,7,8,21,22). CT angiography has a relatively high sensitivity (approximately 94%) and specificity (approximately 85%) for the diagnosis of SAEF (23-25).
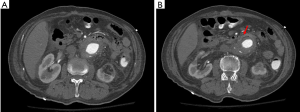
CT findings that strongly suggest SAEF include periaortic foci of gas, periaortic inflammatory changes including fat stranding, periaortic edema, thickening and close proximity of the graft to the adjacent bowel wall, graft thrombosis, perigraft fluid, and extravasation of contrast agent into the GI lumen (6,8,21,26). Several of these findings are nonspecific and can be within normal limits within the first weeks post aortic graft placement, such as foci of gas and perigraft edema. However, if these findings appear several months post-procedure, these signs are suspicious for complication, such as SAEF or endograft infection (24). Figure 1 shows a case of a patient presenting with acute GI bleeding. The value of cross-sectional imaging in the diagnostic work-up of GI bleeding is demonstrated since the CT images revealed an AAA that had formed a fistulous connection with the jejunum.
Open surgical repair of AEF
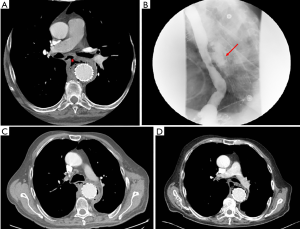
There is a wealth of literature describing open surgical techniques to repair SAEF. Surgical treatment of AEF consists of graft excision with either extra-anatomic bypass or in situ graft reconstruction. With extra-anatomic reconstruction, the bypass can be performed either prior to or simultaneously with the graft excision (27). With in situ reconstruction, the aortic reconstruction is performed immediately after the affected graft is excised. Candidate conduits for aortic reconstruction include a new synthetic graft, arterial homograft, or autogenous femoral venous graft (28). In addition, concomitant surgical repair of the intestinal defect is achieved at the same time. Prior studies have found that, among the aforementioned surgical techniques, in situ repair using a vein graft or prosthetic graft is associated with the lowest long-term mortality rates and lowest rates of sepsis, especially if the graft is covered with omentum. However, even in situ vein grafting for SAEF is associated with an in-hospital mortality rate of 32%, which is considerably higher than the 7% in-hospital mortality of endovascular treatment of SAEF (3,29). Despite decades of attempted improvement and development of novel techniques, morbidity and mortality associated with open surgical repair of SAEF remain major obstacles (28,30-32). Figure 2 presents a 64-year-old man with a prior thoracic aortic aneurysm endograft repair. The patient presented with dysphagia and sharp back pain. CT imaging and esophagogram revealed clearly the presence of an aorto-esophageal fistula which was treated with open surgical repair.
Endovascular repair of AEF
Endovascular repair of SAEF has emerged as an evolving treatment option with potentially improved mortality outcomes.
The principle idea in endovascular stent graft repair of SAEF is to place a stent in order to seal the aortic defect and exclude the fistulous connection to the bowel, thereby stopping the hemorrhage. Typically, this placement involves a significant amount of overlap with the original endograft, as well as extension beyond the original graft edges if an appropriate landing zone is available. At the same time, the graft must not exclude the renal arteries, so placement needs to be highly precise and pre-procedural planning with cross-sectional imaging (usually CT angiography or less likely MR angiography) is of utmost importance. Angiography guided stent graft placement is performed after gaining arterial access via the femoral artery. A guidewire and sheath then guide delivery of the stent graft to the appropriate site, ideally distal to the renal arteries but proximal enough to exclude the fistula (if that is anatomically feasible). Stent graft repair also has the option to be coupled with concomitant endoscopy to repair the intestinal defect at the same time and this may contribute to decreased morbidity and mortality (33).
In addition to stent graft repair, other endovascular techniques have also demonstrated utility in treating SAEFs. Endovascular balloon occlusion can be used to gain rapid control of the aortic bleeding source in a hemodynamically unstable patient. This can gain time to determine a definitive treatment plan (34,35). Similarly, coil embolization can be used as a temporizing or palliative measure to stop bleeding. Coil embolization can also be repeated when further GI bleeding occurs. In a previous study, coil embolization allowed for two to six weeks of bleeding free period before bleeding reoccurred and definitive treatment was required (36). These two to six weeks may help to stabilize the patient in the ICU, thereby potentially improving outcome.
Regardless of what method is used to repair the SAEF, post-procedural contrast-enhanced CT angiography is usually performed to verify closure of the aortic defect. If there are signs of infection around the aneurysmal sac with a sizable fluid collection, CT guided percutaneous drainage can be considered. Broad spectrum antibiotic therapy under guidance of the infectious disease consulting service should be initiated early and maintained chronically to reduce the risk of recurrent sepsis.
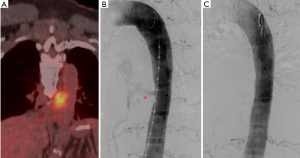
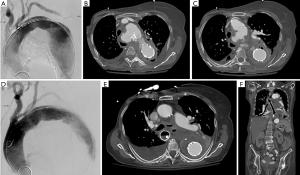
Figure 3 shows a case of a 59-year-old man with esophageal cancer resulting in aorto-esophageal fistula which was treated with an endograft. Figure 4 demonstrates a case of an 85-year-old woman with a history of complicated thoracic aneurysmal disease and subsequent development of aorto-esophageal fistula which was treated by placement of stent-graft into the esophagus.
Outcomes for endovascular treatment of SAEF
Current outcomes for endovascular repair of SAEF are promising. A recent meta-analysis reported an in-hospital mortality rate of 7% for 98 well-documented cases of endovascular SAEF repair from the literature, compared to an in-hospital mortality rate of 34% for the 725 available cases of open surgical SAEF repair. The proportion of endovascular repair patients that did not experience sepsis during the 2-year period following endovascular treatment was remarkably low, at 58%, compared to 81% for the open surgery patients. It needs to be considered that SAEF is a rare disease entity and therefore the number of available cases is relatively limited. Sepsis rate may be improved in the future by using an early preventive broad spectrum antibiotic regimen under the guidance of the infectious disease consulting service and by performing early aggressive percutaneous CT-guided drainage of peri-aortic post-procedure fluid. Additionally, there was no sepsis reported in the eight endovascular repair patients that also had intestinal repair. Overall survival rate after 2 years was still significantly better for those treated with endovascular repair compared to those treated with surgical repair, 51% (endovascular repair) vs. 40% (surgical repair) (3).
Recurrent infection remains a challenging problem following endovascular repair of AEF. Presence of infection pre-procedure was associated with worse outcome after endovascular repair of AEF. The incidence of infection after endovascular repair of AEF with a follow-up of 13 months is estimated to be approximately 44% (37). There has not been a direct comparison of infection associated with open surgical repair versus endovascular repair of AEF to date to the best of our knowledge. The rate of infection in survivors of open surgical repair of AEF after a 9-month follow-up was 25% (38). It is possible that the rate of infection following surgical repair is slightly lower than that following endovascular repair because open surgery allows for debridement of infected tissue and removal of infected grafts. Additionally, there is always increased risk of infection when introducing foreign material. These findings could suggest that when infection is present pre-operatively, endovascular repair may not be ideal as a definitive treatment for AEF, but rather as a bridge to surgery or a stabilizing maneuver. However, more studies are needed to determine and evaluate variables that determine differences in outcome, and to compare outcomes directly between endovascular and open surgical repair of AEF.
It may be beneficial to repair the intestinal defect endoscopically at the same time as endovascular repair of the aorta. Post-procedure broad spectrum antibiotics may lower the sepsis rate as well.
Conclusions
AEF is a rare and potentially life-threatening condition. Early diagnosis and aggressive management of AEF are essential. In this article, less-common types of AEFs, including three aorto-esophageal fistulas and one aorto-jejunal fistula were presented. Additionally, the value of contrast-enhanced CT imaging for timely recognition has been demonstrated based on these cases.
Traditionally treatment of AEF has been associated with high mortality. A variety of open surgical techniques have been described, but intraoperative and in-hospital mortality for surgical repair of AEF remains high. Endovascular repair of AEF has emerged as a promising treatment option with markedly lower short-term mortality compared to open surgery. Most complications after endovascular repair are related to sepsis shortly after the procedure and recurrence months to years post procedure.
AEFs are a rare disease entity and therefore randomized prospective clinical trial to compare different treatment techniques may be challenging to conduct. However, more data regarding specific treatment methods and outcomes are required. Helpful data for every case may include patient characteristics, AEF type and location, treatment modality, exact type of surgical or endovascular procedure, post-procedural management, and short as well as long-term morbidity and mortality. One attractive solution to acquire these data may be a multicenter database registry.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- El Sakka K, Halawa M, Kotze C, et al. Complications of open abdominal aortic surgery: the endovascular solution. Interact Cardiovasc Thorac Surg 2008;7:121-4. [Crossref] [PubMed]
- Chiesa R, Melissano G, Marone EM, et al. Aorto-oesophageal and aortobronchial fistulae following thoracic endovascular aortic repair: a national survey. Eur J Vasc Endovasc Surg 2010;39:273-9. [Crossref] [PubMed]
- Kakkos SK, Bicknell CD, Tsolakis IA, et al. Editor's choice - Management of secondary aorto-enteric and other abdominal arterio-enteric fistulas: a review and pooled data analysis. Eur J Vasc Endovasc Surg 2016;52:770-86. [Crossref] [PubMed]
- Tomlinson MA, Gold B, Thomas MH, et al. Endovascular stent graft repair of a recurrent aorto-enteric fistula. Eur J Vasc Endovasc Surg 2002;24:459-61. [Crossref] [PubMed]
- Danneels MI, Verhagen HJ, Teijink JA, et al. Endovascular repair for aorto-enteric fistula: a bridge too far or a bridge to surgery? Eur J Vasc Endovasc Surg 2006;32:27-33. [Crossref] [PubMed]
- Batt M, Jean-Baptiste E, O'Connor S, et al. Early and late results of contemporary management of 37 secondary aortoenteric fistulae. Eur J Vasc Endovasc Surg 2011;41:748-57. [Crossref] [PubMed]
- Chopra A, Cieciura L, Modrall JG, et al. Twenty-year experience with aorto-enteric fistula repair: gastrointestinal complications predict mortality. J Am Coll Surg 2017;225:9-18. [Crossref] [PubMed]
- Hashimoto M, Goto H, Akamatsu D, et al. Long-term outcomes of surgical treatment with in situ graft reconstruction for secondary aorto-enteric fistula. Ann Vasc Dis 2016;9:173-9. [Crossref] [PubMed]
- Gnus J, Ferenc S, Kościelna M, et al. Secondary aortoenteric fistula after abdominal aortic graft implementation in our own material. Adv Clin Exp Med 2016;25:1265-71. [Crossref] [PubMed]
- Kakkos SK, Antoniadis PN, Klonaris CN, et al. Open or endovascular repair of aortoenteric fistulas? A multicentre comparative study. Eur J Vasc Endovasc Surg 2011;41:625-34. [Crossref] [PubMed]
- Rhéaume P, Labbé R, Thibault E, et al. A rational, structured approach to primary aortoenteric fistula. Can J Surg 2008;51:E125-6. [PubMed]
- Dossa CD, Pipinos II, Shepard AD, et al. Primary aortoenteric fistula: Part I. Ann Vasc Surg 1994;8:113-20. [Crossref] [PubMed]
- Zaki M, Tawfick W, Alawy M, et al. Secondary aortoduodenal fistula following endovascular repair of inflammatory abdominal aortic aneurysm due to Streptococcus anginosus infection: A case report and literature review. Int J Surg Case Rep 2014;5:710-3. [Crossref] [PubMed]
- Schoell T, Manceau G, Chiche L, et al. Surgery for secondary aorto-enteric fistula or erosion (SAEFE) complicating aortic graft replacement: a retrospective analysis of 32 patients with particular focus on digestive management. World J Surg 2015;39:283-91. [Crossref] [PubMed]
- Yazdanpanah K, Minakari M. Intermittent herald bleeding: an alarm for prevention of the exsanguination of aortoenteric fistula before it arrives. Int J Prev Med 2012;3:815-6. [PubMed]
- O'Mara C, Imbembo AL. Paraprosthetic-enteric fistula. Surgery 1977;81:556-66. [PubMed]
- Reilly LM, Ehrenfeld WK, Goldstone J, et al. Gastrointestinal tract involvement by prosthetic graft infection. The significance of gastrointestinal hemorrhage. Ann Surg 1985;202:342-8. [Crossref] [PubMed]
- Valentine RJ, Timaran CH, Modrall GJ, et al. Secondary aortoenteric fistulas versus paraprosthetic erosions: is bleeding associated with a worse outcome? J Am Coll Surg 2008;207:922-7. [Crossref] [PubMed]
- Bunt TJ. Synthetic vascular graft infections. II. Graft-enteric erosions and graft-enteric fistulas. Surgery 1983;94:1-9. [PubMed]
- Leon LR Jr, Psalms SB, Ihnat DM, et al. Aortofemoral graft limb-to-colon paraprosthetic fistula. J Vasc Surg 2008;47:460. [Crossref] [PubMed]
- Hobbs SD, Kumar S, Gilling-Smith GL. Epidemiology and diagnosis of endograft infection. J Cardiovasc Surg (Torino) 2010;51:5-14. [PubMed]
- Hamdani R, Summers R. Vascular-enteric fistula: diagnosis by colonoscopy. Gastrointest Endosc 1995;42:80-1. [Crossref] [PubMed]
- Mark A, Moss AA, Lusby R, et al. CT evaluation of complications of abdominal aortic surgery. Radiology 1982;145:409-14. [Crossref] [PubMed]
- Low RN, Wall SD, Jeffrey RB Jr, et al. Aortoenteric fistula and perigraft infection: evaluation with CT. Radiology 1990;175:157-62. [Crossref] [PubMed]
- Mark AS, Moss AA, McCarthy S, et al. CT of aortoenteric fistulas. Invest Radiol 1985;20:272-5. [Crossref] [PubMed]
- Taheri MS, Haghighatkhah H, Pourghorban R, et al. Multidetector computed tomography findings of abdominal aortic aneurysm and its complications: a pictorial review. Emerg Radiol 2013;20:443-51. [Crossref] [PubMed]
- Bastounis E, Papalambros E, Mermingas V, et al. Secondary aortoduodenal fistulae. J Cardiovasc Surg (Torino) 1997;38:457-64. [PubMed]
- Xiromeritis K, Dalainas I, Stamatakos M, et al. Aortoenteric fistulae: present-day management. Int Surg 2011;96:266-73. [Crossref] [PubMed]
- Oderich GS, Bower TC, Hofer J, et al. In situ rifampin-soaked grafts with omental coverage and antibiotic suppression are durable with low reinfection rates in patients with aortic graft enteric erosion or fistula. J Vasc Surg 2011;53:99-106,107.e1-7; discussion:106-7.
- Speziale F, Rizzo L, Fadda GF, et al. Surgical approach for the treatment of secondary aortoenteric fistulae. Eur J Vasc Endovasc Surg 1998;16:530-4. [Crossref] [PubMed]
- Umpleby HC, Britton DC, Turnbull AR. Secondary arterio-enteric fistulae: a surgical challenge. Br J Surg 1987;74:256-9. [Crossref] [PubMed]
- Reckless JP, McColl I, Taylor GW. Aorto-enteric fistulae: an uncommon complication of abdominal aortic aneurysms. Br J Surg 1972;59:458-60. [Crossref] [PubMed]
- Mok VW, Ting AC, Law S, et al. Combined endovascular stent grafting and endoscopic injection of fibrin sealant for aortoenteric fistula complicating esophagectomy. J Vasc Surg 2004;40:1234-7. [Crossref] [PubMed]
- Loftus IM, Thompson MM, Fishwick G, et al. Technique for rapid control of bleeding from an aortoenteric fistula. Br J Surg 1997;84:1114. [Crossref] [PubMed]
- Leonhardt H, Mellander S, Snygg J, et al. Endovascular management of acute bleeding arterioenteric fistulas. Cardiovasc Intervent Radiol 2008;31:542-9. [Crossref] [PubMed]
- Karkos CD, Vlachou PA, Hayes PD, et al. Temporary endovascular control of a bleeding aortoenteric fistula by transcatheter coil embolization. J Vasc Interv Radiol 2005;16:867-71. [Crossref] [PubMed]
- Antoniou GA, Koutsias S, Antoniou SA, et al. Outcome after endovascular stent graft repair of aortoenteric fistula: A systematic review. J Vasc Surg 2009;49:782-9. [Crossref] [PubMed]
- Bíró G, Szabó G, Fehérvári M, et al. Late outcome following open surgical management of secondary aortoenteric fistula. Langenbecks Arch Surg 2011;396:1221-9. [Crossref] [PubMed]


