May-Thurner: diagnosis and endovascular management
Introduction
In 1957, May and Thurner described the condition by which the chronic pulsations of the overlying right common iliac artery lead to spur formation along the vein wall of the left common iliac vein (CIV) (1). Chronic trauma by these pulsatile forces overtime can lead to accumulation of elastin and collagen with intimal proliferation (2). The anterior-posterior CIV diameter becomes narrow while the transverse diameter widens. The narrowing in the CIV can lead to stasis of venous return within the lower extremity veins. This is a human condition that occurs irrespective of gender or ethnicity. Left CIV compression is often combined with other risk factors such as immobilization, dehydration, surgery, hormones (such as birth control pills, testosterone, and steroids) and genetic factors. Complication of multiple factors increases the risk of deep vein thrombosis (DVT). The incidence of hemodynamically significant compression of the left CIV is thought to be present in 1/3 of the population (3,4). Both genders are equally affected. However, the incidence of May-Thurner syndrome (MTS), involving various degrees of edema, heaviness, and pain in the left leg is unknown, and ranges from 18–49% in patients who present with a left lower extremity DVT (5). The overall relative incidence of the underlying compression is unknown since many patients are asymptomatic. The largest meta-analysis of 1,046 patients who underwent thrombolysis for clot burden within the iliofemoral system revealed an underlying iliac vein compression in 46% of patients (3). Surgical interventions have become obsolete as recent studies have shown that endovascular venous stenting is a safe and effective treatment for iliac venous compression (6-8).
Berger et al. [1995] were the first to describe catheter directed thrombolysis plus stent placement for patients with thrombotic MT (9). In their initial study at six months follow-up, all patients remained asymptomatic and stent remained patent. Since then, multiple studies have looked at the short term and long term patency rates of stent placement in patients with MTS. Almost all of these studies have shown successful primary patency rates of 80–95% with clinical symptomatic improvement (6-8,10).
Clinical presentation
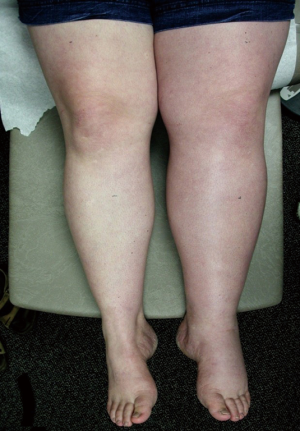
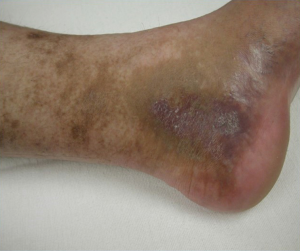
Although many MTS patients present with left lower extremity DVT, symptoms can also include left lower extremity swelling, pain, venous claudication, ulcerations, and varicose veins. Rare symptoms would include phlebitis and phlegmasia cerulean dolens. (8). There are three clinical stages of MTS: (I) asymptomatic left CIV compression; (II) the formation of a venous spur; (III) left lower extremity DVT (6). Actually many patients live with progressive left side venous hypertension and do not recognize it. They have increasing tightness or discomfort with activity, but are better in the morning. Patients can describe that their left shoe may be tighter by the evening. There may be a visible, but small difference in size. On physical examination, patients can have swelling, hyperpigmentation, telangiectasias, or venous ulcerations. Patients, however, often learn to live with these indolent changes and remain underdiagnosed. Therefore good clinical diagnostic evaluation is necessary (Figures 1,2).
Role of CT venography and intravascular ultrasound (IVUS)
Imaging has its role in helping to identify patients who present with signs of MTS; however there are no standardized criteria to establish the diagnosis. Ultrasound is usually the initial modality of choice because it is noninvasive. The IVC/iliac vein exam, however, can be a challenge to perform. Most often patients, who present to the emergency room with left leg pain and/or swelling, are only examined with a lower extremity duplex, which is easy to perform. The lower extremity duplex ultrasound may show no evidence of DVT and the failure to examine the iliac vein segments means MT is frequently missed in these symptomatic patients. Although ultrasound can determine vessel patency and detect iliac venous thrombosis, it can be technically challenging to diagnose the compression of the iliac vein (11). The iliac venous obstruction may result in non-phasic flow in the external iliac vein which serves as a clue to the underlying obstruction or compression. Better data now show that high CIV velocities indicate compression (12). However, these exams are dependent upon technologist experience and patient size.
CT venography is a useful modality. It can identify the compression of the iliac vein; however limitations do exist such as the inability to control for the volume status of the patient during scanning which can lead to overdiagnosis of degree of compression in a dehydrated patient (6). CT is useful in diagnosing and ruling out other causes of left lower extremity swelling. The three most common mimickers of MT are venous obstruction from underlying malignancy related lymphadenopathy, hematoma, or cellulitis (13). In a study by Liu et al., CT venography was found to have a high sensitivity and specificity in confirming MTS over other modalities (14). Although useful, further studies are needed to complete the overall picture.
Few studies have advocated the use of MRV which can estimate the degree of venous collateral flow, and can identify the presence of iliac spurs. However, MRV is expensive, and images do not account for non-laminar venous flow which can occur at bifurcations. McDermott et al. looked at the role that magnetic resonance imaging (MRI)/magnetic resonance venography may play in diagnosing MTS. Their population of 214 patients showed that the compressed left CIV on a single MRV study was not stable over time, and therefore may be insufficient to diagnose MTS (15).
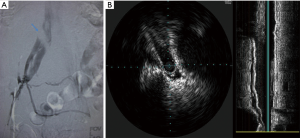
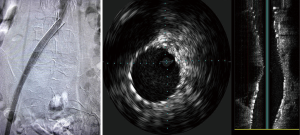
The gold standard for the diagnosis of MTS is conventional venography with IVUS. IVUS has traditionally been a helpful tool in therapeutic interventions involving the coronary and peripheral circulation. In venous studies, specific measurements of luminal area and diameters can be obtained without the requirement of contrast and lateral projection imaging. IVUS is composed of a catheter-mounted transducer with various catheter sizes and transducer frequencies ranging from 12–40 MHz. The information obtained from IVUS can include real-time evaluation of the vessel lumen, the accurate sizing of the luminal diameter and information about the wall itself, i.e., chronic hyperplasia (16). Forauer et al. performed a retrospective review of 16 patients in whom the presumptive diagnosis of MTS was made on cross-sectional imaging and looked at how IVUS influenced endovascular management. Their results showed obliteration of the vein lumen at the level of the crossing artery in every patient, and in 68% of patients, found the level of narrowing to extend to the external iliac vein. Thrombus was also noted in four of their patients (16). Another detail IVUS can provide is the chronicity of the thrombus present. For example, acute hyperechoic thrombus can be identified within the vessel lumen; however, linear filling defects can also be observed representing synechiae or intimal hyperplasia from a more chronic nature. The characterization of thrombus is important as it can guide the decision to perform thrombolysis to dissolve acute clot burden, prior to any further intervention. Another important useful aspect of IVUS is its ability to identify and localize a guidewire in clinical situations where recanalization is performed and multiple channels and perivenous collaterals may be present (16). IVUS is useful for measuring iliac luminal areas and diameters which are important in selecting stent size and length to place the optimal stent for the patient, and thereby minimize chance of stent migration. Finally, IVUS allows for more accurate stent deployment by facilitating accurate identification of the iliac vein confluence. This assists in accurate deployment by reducing the chance that a portion of the stent will unduly obstruct the right CIV (16).
Endovascular stent placement for MTS
Given the “spur” and scar formation that occurs from MTS, it is clear that venous angioplasty is not in itself an effective treatment (17,18). Catheter based strategies have become increasingly important, particularly in the younger patient population, given the high association of iliofemoral thrombosis with post-thrombotic syndrome (PTS). In those patients with acute thrombus, initial catheter-directed thrombolysis is performed. Following clot dissolution, endovascular stents can be placed at the site of iliac vein compression. The first report of stent placement in a patient with thrombotic MTS was by Berger et al. [1995]. Since then, a handful of studies have demonstrated its efficacy (10,17,19). Mewissen et al. showed that a third of patients who were treated with thrombolysis for iliofemoral DVT required stenting and that the stented patients had significantly higher patency than those who were not stented (20). From the resultant studies, the Guidelines by the Society of Interventional Radiology and the Society of Vascular Surgery recommend iliac venous stenting in the setting of external iliac vein compression (21,22).
Options for venous stenting include both stainless steel and nitinol-based stents. Self-expanding stents are preferred. Wallstents (Boston Scientific Corporation, Natick, MA, USA) allow for strength and flexibility with high radial force. However, foreshortening and/or precise placement can be challenging and requires experience. Nitinol stents can be precisely delivered, because there is no foreshortening during deployment. However, they are more prone to compress in the common iliac location. The durability of stents in the iliac veins is described in the setting of DVT, with primary patency rates of 79% at 72 months (23). Hager et al. looked at the mid and long term patency rates of endovascular stents in patients treated with symptomatic and nonthrombotic MTS. Their results were concordant with prior reports showing favorable patency rates; 91% at 36 months in patients who presented determinants of long pain and swelling but no DVT and 91% at 36 months for patients who presented in a post-thrombotic state (10). Stenting was found to be favorable in both groups (10).These findings demonstrate that stent patency is not affected by the ongoing extrinsic compression. Hypercoagulability, residual thrombus affecting overall flow, and anticoagulation may be determinants of long-term patency in some individuals (Figures 3,4).
Conclusions
The diagnosis of MTS is important in order to prevent continued spur formation and associated intimal hyperplasia that can lead to multiple DVTs and ultimately post thrombotic syndrome. It is most commonly associate with the left lower extremity, but in 2–5% it can be bilateral due to a high bifurcation of the aorta resulting in compression of both sides. No current diagnostic criteria are in place to accurately diagnose MTS. A collaborative approach to clinical presentation and imaging findings can help diagnose MTS. Ultimately conventional venography with IVUS remains the most widely used tools to determine appropriate endovascular treatment strategies.
The diagnosis of MTS is unfortunately more associated with DVT than non-thrombotic venous hypertension. This is an unrecognized clinical syndrome in primary care medicine. Those of us in the business of treating DVT recognize that the iliac compression is the culprit lesion that leads to the syndrome of edema and pain or DVT and PTS. The treatment of non-thrombotic iliac compression has only become an option in the past 15 years, since clinical follow-up has shown acceptable patency rates for single iliac vein stents. Wider recognition of MTS by patients and physicians has led to greater demand for both evaluation and endovascular treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- May R, Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology 1957;8:419-27. [Crossref] [PubMed]
- Oğuzkurt L, Ozkan U, Tercan F, et al. Ultrasonographic diagnosis of iliac vein compression (May-Thurner) syndrome. Diagn Interv Radiol 2007;13:152-5. [PubMed]
- Kibbe MR, Ujiki M, Goodwin AL, et al. Iliac vein compression in an asymptomatic patient population. J Vasc Surg 2004;39:937-43. [Crossref] [PubMed]
- Raju S, Neglen P. High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: a permissive role in pathogenicity. J Vasc Surg 2006;44:136-43; discussion 144. [Crossref] [PubMed]
- Kasirajan K, Gray B, Ouriel K. Percutaneous AngioJet thrombectomy in the management of extensive deep venous thrombosis. J Vasc Interv Radiol 2001;12:179-85. [Crossref] [PubMed]
- Ibrahim W, Al Safran Z, Hasan H, et al. Endovascular management of may-thurner syndrome. Ann Vasc Dis 2012;5:217-21. [PubMed]
- Raju S. Treatment of iliac-caval outflow obstruction. Semin Vasc Surg 2015;28:47-53. [Crossref] [PubMed]
- Brinegar KN, Sheth RA, Khademhosseini A, et al. Iliac vein compression syndrome: Clinical, imaging and pathologic findings. World J Radiol 2015;7:375-81. [Crossref] [PubMed]
- Berger A, Jaffe JW, York TN. Iliac compression syndrome treated with stent placement. J Vasc Surg 1995;21:510-4. [Crossref] [PubMed]
- Hager ES, Yuo T, Tahara R, et al. Outcomes of endovascular intervention for May-Thurner syndrome. J Vasc Surg Venous Lymphat Disord 2013;1:270-5. [Crossref] [PubMed]
- Patel NH, Stookey KR, Ketcham DB, et al. Endovascular management of acute extensive iliofemoral deep venous thrombosis caused by May-Thurner syndrome. J Vasc Interv Radiol 2000;11:1297-302. [Crossref] [PubMed]
- Metzger PB, Rossi FH, Kambara AM, et al. Criteria for detecting significant chronic iliac venous obstructions with duplex ultrasound. J Vasc Surg Venous Lymphat Disord 2016;4:18-27. [Crossref] [PubMed]
- Wu WL, Tzeng WS, Wu RH, et al. Comprehensive MDCT evaluation of patients with suspected May-Thurner syndrome. AJR Am J Roentgenol 2012;199:W638-45. [Crossref] [PubMed]
- Liu Z, Gao N, Shen L, et al. Endovascular treatment for symptomatic iliac vein compression syndrome: a prospective consecutive series of 48 patients. Ann Vasc Surg 2014;28:695-704. [Crossref] [PubMed]
- McDermott S, Oliveira G, Ergül E, et al. May-Thurner syndrome: can it be diagnosed by a single MR venography study? Diagn Interv Radiol 2013;19:44-8. [PubMed]
- Forauer AR, Gemmete JJ, Dasika NL, et al. Intravascular ultrasound in the diagnosis and treatment of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol 2002;13:523-7. [Crossref] [PubMed]
- Park JY, Ahn JH, Jeon YS, et al. Iliac vein stenting as a durable option for residual stenosis after catheter-directed thrombolysis and angioplasty of iliofemoral deep vein thrombosis secondary to May-Thurner syndrome. Phlebology 2014;29:461-70. [Crossref] [PubMed]
- Nazarian GK, Bjarnason H, Dietz CA Jr, et al. Iliofemoral venous stenoses: effectiveness of treatment with metallic endovascular stents. Radiology 1996;200:193-9. [Crossref] [PubMed]
- Jeon UB, Chung JW, Jae HJ, et al. May-Thurner syndrome complicated by acute iliofemoral vein thrombosis: helical CT venography for evaluation of long-term stent patency and changes in the iliac vein. AJR Am J Roentgenol 2010;195:751-7. [Crossref] [PubMed]
- Mewissen MW, Seabrook GR, Meissner MH, et al. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology 1999;211:39-49. [Crossref] [PubMed]
- Vedantham S, Millward SF, Cardella JF, et al. Society of Interventional Radiology position statement: treatment of acute iliofemoral deep vein thrombosis with use of adjunctive catheter-directed intrathrombus thrombolysis. J Vasc Interv Radiol 2006;17:613-6. [Crossref] [PubMed]
- Meissner MH, Gloviczki P, Comerota AJ, et al. Early thrombus removal strategies for acute deep venous thrombosis: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg 2012;55:1449-62. [Crossref] [PubMed]
- Neglén P, Hollis KC, Olivier J, et al. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg 2007;46:979-90. [Crossref] [PubMed]


