Acute aortic syndromes and aortic emergencies
Introduction
The term acute aortic syndrome (AAS) encompasses three pathological entities; aortic dissection (AD; 85–95% of AAS), penetrating aortic ulcer (PAU; 2–7%) and intramural hematoma (IMH; 0–25%) (1). Even though other conditions that represent aortic emergencies like trauma, iatrogenic/septic pseudoaneurysms, and ruptured atherosclerotic aneurysm have similar signs and symptoms, the term AAS is typically reserved for the aforementioned triad of AD, PAU and IMH entities. These conditions have a common clinical presentation and are typically associated with acute chest or back pain (2). These conditions are relatively uncommon (AAS incidence of 3.5 to 6.0 per 100,000 patient/years); but carry a high mortality rate (80–90% for aortic trauma and 50–60% for type A AD) (3-5). Rapid, accurate recognition and classification of AAS and aortic emergencies is, therefore, necessary (1,6). Treatment often entails a multidisciplinary approach involving medical, endovascular and surgical interventions. Imaging plays a key role in timely diagnosis and treatment planning.
Terminology and current understanding of AAS and aortic emergencies
AAS/aortic emergencies are often described by a large number of terms (Figures 1,2) that include traumatic and non-traumatic conditions. It is important to understand the terminology since they indicate different distinct pathological and survival implications.
AAS
The underlying etiology of AAS is most often an atherosclerotic disease. However, it can also result from other conditions such as medial degeneration, trauma or infection. Disruption of media layer of the aortic wall is a common feature of AD, IMH, PAU. AD results from splitting of the aortic wall layers by the blood entering through an entrance tear. An intimal flap separates the true lumen (TL), which is continuous with the nondissected aortic segment (unless the whole aorta is dissected), from the false lumen (FL). IMH was initially considered as bleeding within the media secondary to rupture of vasa-vasorum, but this theory has not been thoroughly validated (7). Increasing number of investigations have demonstrated the presence of micro tears, early in the disease course of IMH, by imaging and in pathological specimens (7-10). These findings suggest a common pathophysiologic mechanism between AD and IMH, with the only difference between the two is the presence of a re-entrance tear large enough to maintain the patency of a FL in the AD (Figure 3) (7-9,11). PAU refers to an atherosclerotic plaque that corrodes through the internal elastic lamina into the media to variable depths. It may further spread through the media to form a typical IMH or through the adventitia to form a pseudoaneurysm (12).
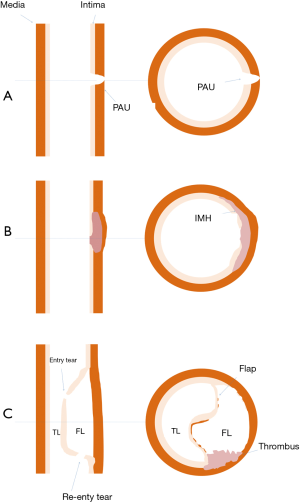
Besides the classic triad, some authors also include limited or focal dissection within the umbrella of AAS. A limited dissection indicates an intimomedial tear without intramural separation (12).
Intimal tear
It refers to focal discontinuity in the internal elastic lamina of the blood vessel that forms the gateway to let blood to spread through the vascular media. Often, these tears are the index events in AAS.
Aortic pseudoaneurysms
This is a blood-filled outpouching that arises from a vessel and does not contain all three layers of the vessel wall (intima, media, and adventitia). It occurs as a consequence of full thickness vessel wall injury or tear. Pseudoaneurysms can form secondary to atherosclerosis, trauma, infection and iatrogenic insult such as aortic cannulation or inadvertent aortic puncture.
Aortic trauma
Total of 75–80% of thoracic aortic injuries are attributed to a rapid deceleration in the setting of high-speed motor vehicle collisions. Aortic root and isthmus are the most vulnerable sites for these injuries since they are the tether points where a relatively mobile aortic arch connects with relatively fixed structures, heart, and descending aorta respectively. The aorta may also be injured directly as a result of penetrating thoracic trauma.
Aortic injuries result in rapid exsanguinations and in most cases fatal before medical attention can be sought. Studies suggest that only 20% patients with an acute traumatic aortic injury initially survive for more than one hour (6,13,14). Nevertheless, a significant number of aortic injuries make their way to the hospital and require prompt diagnosis and management, as if left undetected and untreated they result in rapid in-hospital mortality (30% within the first 6 hours, 49% within the first 24 hours) (10).
Aortic trauma may manifest as limited or classic dissection, IMH, pseudoaneurysms or aortic transection. Aortic transection refers to a full thickness tear of the aortic wall with loss of continuity at the transected ends. This disruption leads to the development of a surrounding hematoma which, depending on its extent, can either remain contained within the mediastinal fat or freely communicate with the pericardial or pleural cavities.
Ruptured aortic aneurysm
Aortic aneurysms most commonly occur as a consequence of atherosclerotic disease. It results from weakening of the diseased aortic wall that is also at an increased risk of rupture. Aneurysms can have an emergent presentation in the following settings; impending rupture, small contained leaks with subtle infiltration of adjacent fat or overt extravasation into the body cavity (Figure 4) (15).
Retrograde type A dissection
Retrograde type A dissection comprises of 7–25% of acute type A dissections which differ from a typical type A dissection with a primary tear in the aorta beyond the left subclavian and the FL propagates in a direction opposite to the direction of blood flow, i.e., towards the proximal arch and ascending aorta. While most acute type A dissections have a tear in the aortic root or ascending aorta with an antegrade propagation of the FL (16,17). This tends to occur in type B dissections when the primary tear is in close proximity to the aortic arch and decompression of FL, through the distal aortic branches, is less effective (17). Compared with antegrade type A, the aortic root is less commonly involved in retrograde type A dissections, and successful aortic valve resuspension is often possible (17).
Prognostication and, triage in AAS and aortic emergencies
Stanford classification is the most widely used prognostic classification for AAS. Although primarily used for ADs, it can be also applied in IMH (12). It divides dissections depending upon segments of aortic involvement. Type A affects ascending aorta and arch and may result in occlusion of coronary vessels, incompetence of aortic valve or rupture into the pericardial space. Type A AAS (AD, IMH, and PAU) and aortic emergencies carry a high risk of mortality with conservative management and are treated with emergent surgical intervention (1,5,18-20). Type B abnormalities commence distal to the origin of the left subclavian artery and uncomplicated type B ADs can be managed by a more conservative approach with medication and/or EVAR, due to a significantly higher mortality after open repair (1,4,21,22). Complicated type B dissections with organ or limb malperfusion, progressive dissection, intractable pain, or uncontrolled hypertension are managed by a more invasive approach, preferably EVAR (5). The goal of EVAR in AD is to seal the entry tear in order to decompress the FL. Fenestration is another treatment option in situations where the TL gets compressed by a high-pressured FL resulting in visceral hypoperfusion, and EVAR stent-graft placement is not feasible. This includes cases with large aortic diameter, a critical vessel close to entry tear or unfavorable anatomy with inadequate seal. Fenestration procedure is performed to create a hole in the dissection flap, which allows outflow from the FL. It decompresses the FL, reduces the risk of dissection flap advancement, and relieves the obstruction of branch vessels. Fenestration procedure may also be combined with stent-graft placement (23). Other indications for an invasive approach in type B include, IMH with enlargement of aortic diameter, development of an ulcer-like projection, or progression of IMH into frank dissection (12). EVAR may also be the treatment of choice in aortic trauma that involves the junction of an arch and the descending thoracic aorta (24).
Imaging of AAS/aortic emergencies
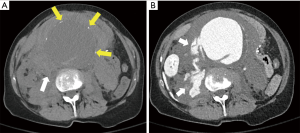
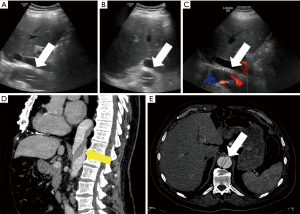
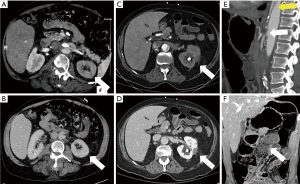
Usually the first imaging modality performed is a chest radiograph. A supine chest radiograph is frequently obtained in the setting of acute trauma to identify life-threatening conditions such as massive hemothorax or tension pneumothorax. It also provides an opportunity to detect unsuspected aortic trauma. A chest radiograph is also frequently obtained in patients who present with chest pain. Contrast-enhanced computed tomography angiography (CTA) is indicated when the radiograph is positive or equivocal in the setting of a high clinical suspicion. CTA is an excellent tool for triaging AAS into surgical and non-surgical management strategies. It can also reliably identify clinically significant aortic injury and ruptured aortic aneurysms in stable patients (24). Since it is rapid, non-invasive, widely available, and provides high quality three-dimensional images, CTA is the diagnostic modality of choice for aortic emergencies and AAS. Transesophageal echocardiography (TEE) and abdominal ultrasound are bedside tools that may enable identification of AAS and aortic emergencies for clinically unstable patients in intensive care units (25). However, TEE requires an experienced operator, sedation, and endotracheal intubation and provides only a limited view of the aortic arch, distal ascending aorta and abdominal aorta. Abdominal aortic aneurysms and dissections may occasionally be detected on abdominal ultrasound examination (Figure 5) in lean patients. However, it is not the imaging modality of choice in diagnosis or long-term follow up for these conditions (25). Magnetic resonance angiography (MRA) can be used as an alternative to CTA in select cases when ionizing radiation or allergic reactions to iodinated intravenous contrast agents are to be avoided (26). Non-contrast MRA can also be performed in patients with renal dysfunction (26). The strengths and weaknesses of different imaging modalities have been summarized in Table 1. Since CTA is the work-horse for the imaging in aortic emergencies/AAS and MRA is the most commonly used alternative imaging the next section is focused on these two modalities.

Full table
CTA
CTA is excellent in defining vascular anatomy and classifying AAS into Stanford types (Figure 6), which is the key to management (27). Variations in anatomy such as retroesophageal anomalous arch vessels, coarctation, right-sided arch and vascular rings can also be recognized.
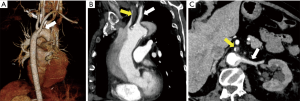
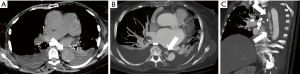
CTA protocol-A typical triple phase CTA protocol is similar to routine aortic angiography and described in detail in the article on non-invasive aortic imaging in the same issue. It is important to re-emphasize that a non-contrast phase is essential in patients with suspected AAS because IMH and wall calcifications are apparent on this phase (Figure 7). Cardiac motion can result in artifacts which may mimic or obscure dissection flaps in the proximal ascending aorta. Motion also obscures the anatomical details of the aortic root (21,22). To overcome these limitations aortic CTA is performed with ECG-gated protocols which minimize pulsation artifacts and dramatically improve visualization of the aortic root and ascending aorta (28,29). Examination of the thoracic aorta should always include the evaluation of abdominal aorta and iliac arteries, because of the high risk of involvement of these vessels and also to facilitate endovascular treatment planning, if needed. Delayed phase is acquired 1 to 2 minutes after injection to assess late filling of contrast in the FL (Figure 8), contrast extravasation in aortic rupture and end organ hypoperfusion (Figure 9) (30-35). It is also often required for evaluation of associated abdominal vascular and visceral injuries in trauma (30-35). A cerebrovascular accident in a setting of AD carries a poor prognosis (25), therefore CT and CTA of the head must be acquired in cases with suggestive symptoms or if the innominate or left carotid artery is involved.
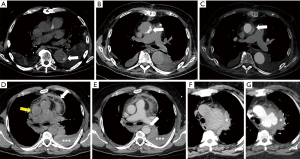
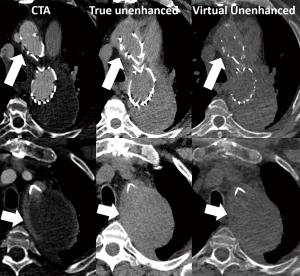
CTA protocols performed with dual-energy CT (DECT) technique provide additional benefits in vascular imaging. While a standard triple-phase CTA entails acquisition of a true non-contrast (TNC) phase, virtual-unenhanced images can be created from a DECT, thus enabling streamlined protocols with considerably reduced radiation exposure (Figure 10) (36). Furthermore, availability of different virtual monochromatic and material-density image reconstructions can be exploited to assess the vascular and parenchymal enhancement or to mitigate artifacts secondary to metal hardware. These patients may have compromised hemodynamic status, the improved contrast-to-noise ratio of low keV images can also be exploited to reduce the amount of contrast media administered (37,38).
In addition to assessing for AAS, CT also enables in establishing an alternate diagnosis or depicts other incidental findings. Incidental findings are routinely detected (up to 89%) during CTA and when significant must be communicated in the report (39).
MRA
The urgency of diagnosis AAS, limited availability of MRA during afterhours and the high average scan time limits its use as the diagnostic modality of choice. Contrast-enhanced MRA can be a useful alternative to CTA in selected patients with iodinated contrast allergy and when ionizing radiation has to be avoided (26). Non-contrast MRA can also be performed in patients with renal failure where use of intravenous contrast media is contraindicated (26).
Rapid black blood sequences provide a natural contrast between the lumen and the vessel wall layers. A standard MRA should begin with T1W and T2W black blood sequences covering the entire aorta (40). A non-ECG gated fluoroscopically triggered gadolinium (0.2 mmol/kg of body weight) enhanced MRA can be acquired over a period of few minutes in severely ill patients within a matter of seconds (although setting up the exam takes much longer time and additional sequences are required for full interpretation). An ECG tracing can be acquired with the imaging data, which can be reconstructed in the different phases of the cardiac cycle and displayed in cine mode. Non-contrast MRA with balanced steady-state free precession techniques are increasingly applied in patients at risk for renal insufficiency or nephrogenic systemic fibrosis (25,41).
As the protocols for MR are evolving and acquisitions with shorter scan times are being explored, the use of MR in the emergency department is increasing (26). A recent study demonstrated that majority of MRIs performed in the emergency department do not increase the length-of-stay (42). This has implications on the existing workflow and reassessment of utilization of MR in this setting for different indications, especially vascular emergencies.
Imaging signs for AAS/aortic emergencies
AAS
As discussed in the definition section AAS probably start with a breach in the intima. Intimal tears are well defined in AD but may be difficult to visualize in IMH and PAU.
PAU
On CTA and MRA a penetrating ulcer is often seen as an irregular ulcerated plaque with contrast agent outpouching extending beyond the aortic intima with variable degree of associated IMH.
IMH
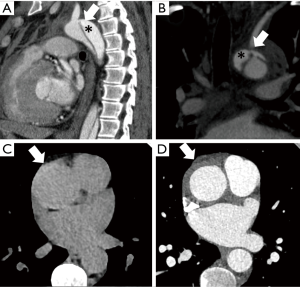
The IMH is identified as a crescentic thickening of the aortic wall containing clotted blood products. These blood products are hyperdense on CT and are associated with inward displacement of the aortic intima. Intimal calcification may help in defining the vessel intima (43). Both of these findings can be appreciated on non-contrast CT images. IMH may also appear hyperintense on T1W black-blood images on MRA. Imaging findings that prognosticate IMH include Stanford type, IMH thickness, maximal aortic diameter, and presence of ulcer-like projections on adventitial aspect and intramural blood pools. The intramural blood pool in the IMH is classically described as Chinese ring sword sign (Figure 11) (44).
AD
The hallmark of the AD is a flap that divides the aorta into true and FL. Radiographs may rarely show displacement of intimomedial calcification at the aortic knuckle with AAS or AD; and often may be negative or show non-specific secondary signs such as pleural effusion. The important clinical information in AD can be classified under following headings.
Site of injury
CTA is highly accurate in depicting site of intimal tear and Stanford type classification and branch vessel involvement.
Extent of involvement
Relationship of the origins of branch vessels relative to true versus FL is particularly important for surgical planning. The degree of involvement of arch vessels may influence the surgical approach and prognosis in type A dissection and should be addressed while assessing aortic CTA (25). Extension into the aortic root (dysfunction of the aortic valve), and coronaries must also be looked for in type A dissection. Left-sided branches of the abdominal aorta (most often the left renal artery) are at a higher risk of involvement compared to the right side (25). AD may influence the flow through a branch vessel in a number of ways. Branch can originate from a FL with limited flow or thrombosis, vessel can arise from upstream obstructed TL, a flap can cause by dynamic obstruction of flow prolapse into the ostium or fixed obstruction by stopping at or extending into the vessel (25,29). The signs of these mechanisms must be looked for on CTA. Contrast enhanced MRA has similar sensitivity for detection of AD flap, anatomical classification and branch vessel involvement. Additionally, gradient-echo sequences or phase contrast images can be acquired in stable patients for identifying entry or re-entry sites, aortic insufficiency and differentiating slow flow from thrombus in the FL of patients with AD (45-47).
TEE can identify dissection flaps, localize the intimal tear, detect IMH, and atherosclerotic PAU in sick patients in ICU setting (48). It performs better for the intimal flap in the proximal ascending aorta and a short segment of the descending aorta but often limited in evaluation of the arch and distal descending aorta (25). TEE is useful in identifying aortic valve dysfunction, pericardial tamponade, or wall motion abnormalities. It may also add additional value in depicting the occurrence and mechanism of aortic regurgitation secondary to AD. Aortic regurgitation manifests when the dissection flap extends into the sinus of Valsalva or prolapse through the aortic orifice. Identification of mechanism helps in determining the mode of surgical correction and may facilitate root sparing repair (49). It is also useful in determining the origins of the coronary ostia in relation to dissection flap, extension of flap into the coronary, ventricular function, pericardial effusion and tamponade (25).
Secondary complications
Pericardial or pleural effusion, in association with dissection, should raise the suspicion of rupture into the pericardial or pleural space. Pericardial fluid with an attenuation of over 40 Hounsfield units (HU) on non-contrast scan should be considered as hemopericardium (50). Involvement of visceral vasculature is a risk factor for mortality and is better evaluated by performing venous or delayed phases as part of CTA (51). CT also depicts findings of altered visceral perfusion that occur as consequence of aortic emergencies. Since left branch vessels are most commonly involved, the left kidney carries the greatest risk of visceral ischemia. On imaging, this is visualized as areas of relative hypoenhancement as compared to remainder ipsilateral or contralateral kidney. Involvement of bilateral intercostal arteries may result in spinal medullary infarct. In approximately one quarter of patients, AD is associated with lower limb ischemia (25).
Aortic trauma
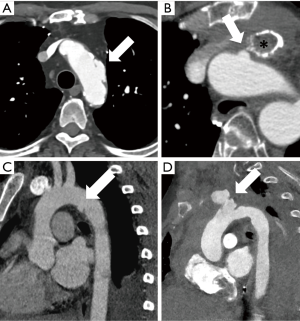
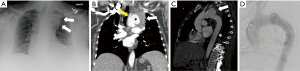
Aortic injury may range from intimal injury alone (minimal aortic injury) to aortic transection with active extravasation (Figure 2). The aortic isthmus is the most common site of insult (52). These injuries often occur along the medial curvature of the distal arch close to the left pulmonary artery and left main stem bronchus. Mediastinal widening greater than 8 cm or 25% of the width of the thorax is the most frequent and sensitive radiographic finding for mediastinal hematoma (Figure 12) (6,53,54). Presence of contour abnormality of the transverse aortic arch, loss of aortopulmonary window and obscuration of the interface between the lung and transverse or descending thoracic aorta also raise concern for significant aortic trauma. Secondary signs, like depression of left main stem bronchus, rightward deviation of tracheal or nasogastric tubes, left apical cap, fractures of the first and second ribs, hemothorax and pneumothorax, may also indicate the presence of an aortic injury (6). It should be borne in mind that up to 7% of aortic trauma cases have normal chest radiographs (53). Therefore, a high index of suspicion such as in patients with a history of rapid deceleration injury warrants further evaluation despite a normal radiograph. Furthermore, these findings can also be seen in conditions such as goiter, mediastinal masses leading to false positive outcomes. Therefore, when aortic trauma is suspected on a radiograph in stable patients, it should always be evaluated further by CTA (55). In unstable patients, an emergent thoracotomy may be indicated.
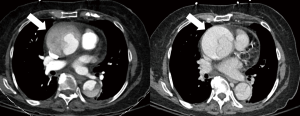
A soft-tissue-attenuation mass with areas of high attenuation (>60 HU), associated with loss of periaortic fat planes on CTA should raise a high suspicion for aortic trauma. Other findings include a sudden change in the aortic caliber, abnormal aortic contour, intimal flap, pseudoaneurysm and intraluminal mural thrombus (6). Multiple autopsy series have reported ascending aorta and root as the second most common location for aortic trauma (56). However, infliction at this location is rarely seen on CTA, presumably due to high risk of pre-hospital demise (57). Injuries to the aortic arch and branch vessels are less common but potentially fatal (Figure 13).
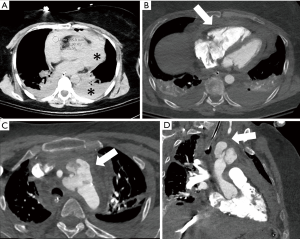
Normal post-isthmic aortic dilatation or aortic spindle and ductus remnants are a normal finding in the isthmic region and should not be misinterpreted as an aortic injury. Collapsed lung adjacent to the aorta should also not be mistaken as intimal flap or IMH.
Aortic aneurysm rupture
Active contrast extravasation from an aortic aneurysm is diagnostic of aneurysmal rupture (Figure 4). It is generally associated with a variable degree of mediastinal or retroperitoneal hematoma. Contrast material extravasation into the involved portion of the bowel, intraluminal and periaortic extraluminal gas are rare signs of abdominal aortic rupture fistulizing with the bowel (15). The enlarging maximum diameter is the most reliable and established predictive sign of impending aneurysmal rupture, but it requires prior baseline comparison, which might not be available many instances. The additional imaging signs of impending aortic rupture include periaortic fat stranding, draping aorta sign, focal discontinuity in circumferential wall calcifications and hyperdense peripheral crescent within the mural thrombus (15). The draping aorta sign is considered present when the posterior wall of the aortic aneurysm drapes or moulds to the anterior surface of the vertebra. Usually, the fat planes between the aneurysm and vertebra are lost (15).
Conclusions
AAS/aortic emergencies are life threatening conditions that warrant prompt management. It is important to be familiar with the terminology used in the description of AAS/aortic emergencies and their respective implications. Radiology plays a highly valuable role in the management AAS and aortic emergencies. Radiographs are easily obtainable and often the first imaging modality performed. CTA is the most widely used imaging technique. It is quick to perform, provides an excellent definition of anatomy and extent, and detects complications, predictors of progression and end organ ischemia. In select patients with allergy to iodinated contrast or renal dysfunction, MRA can be performed as an alternate to CTA.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Morris JH, Mix D, Cameron SJ. Acute Aortic Syndromes: Update in Current Medical Management. Curr Treat Options Cardiovasc Med 2017;19:29. [Crossref] [PubMed]
- Lansman SL, Saunders PC, Malekan R, et al. Acute aortic syndrome. J Thorac Cardiovasc Surg 2010;140:S92-7. [Crossref] [PubMed]
- Howard DP, Banerjee A, Fairhead JF, et al. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation 2013;127:2031-7. [Crossref] [PubMed]
- Mussa FF, Horton JD, Moridzadeh R, et al. Acute Aortic Dissection and Intramural Hematoma: A Systematic Review. JAMA 2016;316:754-63. [Crossref] [PubMed]
- Nienaber CA, Powell JT. Management of acute aortic syndromes. Eur Heart J 2012;33:26-35b. [Crossref] [PubMed]
- Steenburg SD, Ravenel JG, Ikonomidis JS, et al. Acute Traumatic Aortic Injury: Imaging Evaluation and Management. Radiology 2008;248:748-62. [Crossref] [PubMed]
- Nakashima M, Kaji S, Murai R, et al. Detection of Micro Intimal Tear at a Very Early Stage in Patients With Acute Aortic Intramural Hematoma. Circulation 2016;134:A13989.
- Song JK. Diagnosis of aortic intramural haematoma. Heart 2004;90:368-71. [Crossref] [PubMed]
- Bozzani A, Palmieri P, Arici V, et al. Echo-Free Space and Intimal Micro-Tear: Initiating Event or Decompression Rent of Intramural Haematoma? EJVES Extra 2009;17:17-9. [Crossref]
- Williams JS, Graff JA, Uku JM, et al. Aortic injury in vehicular trauma. Ann Thorac Surg 1994;57:726-30. [Crossref] [PubMed]
- Kan CB, Chang RY, Chang JP. Optimal initial treatment and clinical outcome of type A aortic intramural hematoma: a clinical review. Eur J Cardiothorac Surg 2008;33:1002-6. [Crossref] [PubMed]
- Gutschow SE, Walker CM, Martínez-Jiménez S, et al. Emerging Concepts in Intramural Hematoma Imaging. Radiographics 2016;36:660-74. [Crossref] [PubMed]
- Feczko JD, Lynch L, Pless JE, et al. An autopsy case review of 142 nonpenetrating (blunt) injuries of the aorta. J Trauma 1992;33:846-9. [Crossref] [PubMed]
- Parmley LF, Mattingly TW, Manion WC, et al. Nonpenetrating Traumatic Injury of the Aorta. Circulation 1958;17:1086-101. [Crossref] [PubMed]
- Rakita D, Newatia A, Hines JJ, et al. Spectrum of CT Findings in Rupture and Impending Rupture of Abdominal Aortic Aneurysms. Radiographics 2007;27:497-507. [Crossref] [PubMed]
- Nauta FJ, Tolenaar JL, Patel HJ, et al. Impact of Retrograde Arch Extension in Acute Type B Aortic Dissection on Management and Outcomes. Ann Thorac Surg 2016;102:2036-43. [Crossref] [PubMed]
- DiMusto PD, Rademacher BL, Philip JL, et al. Acute retrograde type A aortic dissection: morphologic analysis and clinical implications. J Surg Res 2017;213:39-45. [Crossref] [PubMed]
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897-903. [Crossref] [PubMed]
- Kawabori M, Kaneko T. Acute aortic syndrome: A systems approach to a time-critical disease. Best Pract Res Clin Anaesthesiol 2016;30:271-81. [Crossref] [PubMed]
- Silaschi M, Byrne J, Wendler O. Aortic dissection: medical, interventional and surgical management. Heart 2017;103:78-87. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol 2010;55:e27-129. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. [Crossref] [PubMed]
- Hartnell GG, Gates J. Aortic Fenestration: A Why, When, and How-to Guide. Radiographics 2005;25:175-89. [Crossref] [PubMed]
- Fox N, Schwartz D, Salazar JH, et al. Evaluation and management of blunt traumatic aortic injury: A practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg 2015;78:136-46. [Crossref] [PubMed]
- Baliga RR, Nienaber CA, Bossone E, et al. The Role of Imaging in Aortic Dissection and Related Syndromes. JACC Cardiovasc Imaging 2014;7:406-24. [Crossref] [PubMed]
- Wang GX, Hedgire SS, Le TQ, et al. MR angiography can guide ED management of suspected acute aortic dissection. Am J Emerg Med 2017;35:527-30. [Crossref] [PubMed]
- McMahon MA, Squirrell CA. Multidetector CT of Aortic Dissection: A Pictorial Review. Radiographics 2010;30:445-60. [Crossref] [PubMed]
- Alkadhi H, Bettex D, Wildermuth S, et al. Dynamic Cine Imaging of the Mitral Valve with 16-MDCT: A Feasibility Study. AJR AJR Am J Roentgenol 2005;185:636-46. [Crossref] [PubMed]
- Johnson TR, Nikolaou K, Becker A, et al. Dual-source CT for chest pain assessment. Eur Radiol 2008;18:773-80. [Crossref] [PubMed]
- Kumar R, Kumar A, Baliyan V, et al. Correlating MDCT Liver Injury Grade and Clinical Outcome in Patients Without Significant Extra-hepatic Injury. Indian J Surg 2016;78:275-80. [Crossref] [PubMed]
- Boscak AR, Shanmuganathan K, Mirvis SE, et al. Optimizing Trauma Multidetector CT Protocol for Blunt Splenic Injury: Need for Arterial and Portal Venous Phase Scans. Radiology 2013;268:79-88. [Crossref] [PubMed]
- Eichler K, Marzi I, Wyen H, et al. Multidetector computed tomography (MDCT): simple CT protocol for trauma patient. Clin Imaging 2015;39:110-5. [Crossref] [PubMed]
- Scheske JA, Chung JH, Abbara S, et al. Computed Tomography Angiography of the Thoracic Aorta. Radiol Clin North Am 2016;54:13-33. [Crossref] [PubMed]
- Hinzpeter R, Boehm T, Boll D, et al. Imaging algorithms and CT protocols in trauma patients: survey of Swiss emergency centers. Eur Radiol 2017;27:1922-8. [Crossref] [PubMed]
- Baghdanian AH, Armetta AS, Baghdanian AA, et al. CT of Major Vascular Injury in Blunt Abdominopelvic Trauma. Radiographics 2016;36:872-90. [Crossref] [PubMed]
- Parakh A, Patino M, Sahani DV. Spectral CT/Dual-Energy CT. In: Medical Radiology. Berlin Heidelberg: Springer, 2017:1-21.
- Shuman WP, O’Malley RB, Busey JM, et al. Prospective comparison of dual-energy CT aortography using 70% reduced iodine dose versus single-energy CT aortography using standard iodine dose in the same patient. Abdom Radiol (NY) 2017;42:759-65. [Crossref] [PubMed]
- Shuman WP, Chan KT, Busey JM, et al. Dual-energy CT Aortography with 50% Reduced Iodine Dose Versus Single-energy CT Aortography with Standard Iodine Dose. Acad Radiol 2016;23:611-8. [Crossref] [PubMed]
- Prabhakar AM, Le TQ, Abujudeh HH, et al. Incidental findings and recommendations are common on ED CT angiography to evaluate for aortic dissection. Am J Emerg Med 2015;33:1639-41. [Crossref] [PubMed]
- Stemerman DH, Krinsky GA, Lee VS, et al. Thoracic aorta: rapid black-blood MR imaging with half-Fourier rapid acquisition with relaxation enhancement with or without electrocardiographic triggering. Radiology 1999;213:185-91. [Crossref] [PubMed]
- Oliveira IS, Hedgire SS, Li W, et al. Blood pool contrast agents for venous magnetic resonance imaging. Cardiovasc Diagn Ther 2016;6:508-18. [Crossref] [PubMed]
- Sánchez Y, Yun BJ, Prabhakar AM, et al. Magnetic Resonance Imaging Utilization in an Emergency Department Observation Unit. West J Emerg Med 2017;18:780-4. [Crossref] [PubMed]
- Kazerooni EA, Bree RL, Williams DM. Penetrating atherosclerotic ulcers of the descending thoracic aorta: evaluation with CT and distinction from aortic dissection. Radiology 1992;183:759-65. [Crossref] [PubMed]
- Wu MT, Wang YC, Huang YL, et al. Intramural Blood Pools Accompanying Aortic Intramural Hematoma: CT Appearance and Natural Course. Radiology 2011;258:705-13. [Crossref] [PubMed]
- Sakuma H, Bourne MW, O’Sullivan M, et al. Evaluation of thoracic aortic dissection using breath-holding cine MRI. J Comput Assist Tomogr 1996;20:45-50. [Crossref] [PubMed]
- Powell AJ, Maier SE, Chung T, et al. Phase-velocity cine magnetic resonance imaging measurement of pulsatile blood flow in children and young adults: in vitro and in vivo validation. Pediatr Cardiol 2000;21:104-10. [Crossref] [PubMed]
- Silverman JM, Raissi S, Tyszka JM, et al. Phase-contrast cine MR angiography detection of thoracic aortic dissection. Int J Card Imaging 2000;16:461-70. [Crossref] [PubMed]
- Evangelista A, Mukherjee D, Mehta RH, et al. Acute intramural hematoma of the aorta: a mystery in evolution. Circulation 2005;111:1063-70. [Crossref] [PubMed]
- Bossone E, Evangelista A, Isselbacher E, et al. Prognostic role of transesophageal echocardiography in acute type A aortic dissection. Am Heart J 2007;153:1013-20. [Crossref] [PubMed]
- Sebastià C, Pallisa E, Quiroga S, et al. Aortic dissection: diagnosis and follow-up with helical CT. Radiographics 1999;19:45-60. [Crossref] [PubMed]
- Vernhet H, Serfaty JM, Serhal M, et al. Abdominal CT Angiography Before Surgery as a Predictor of Postoperative Death in Acute Aortic Dissection. AJR Am J Roentgenol 2004;182:875-9. [Crossref] [PubMed]
- Dosios TJ, Salemis N, Angouras D, et al. Blunt and penetrating trauma of the thoracic aorta and aortic arch branches: an autopsy study. J Trauma 2000;49:696-703. [Crossref] [PubMed]
- Fabian TC, Richardson JD, Croce MA, et al. Prospective study of blunt aortic injury: multicenter trial of the American Association for the Surgery of Trauma. J Trauma 1997;42:374-80; discussion 380-3. [Crossref] [PubMed]
- Marnocha KE, Maglinte DD, Woods J, et al. Mediastinal-width/chest-width ratio in blunt chest trauma: a reappraisal. AJR Am J Roentgenol 1984;142:275-7. [Crossref] [PubMed]
- von Kodolitsch Y, Nienaber CA, Dieckmann C, et al. Chest radiography for the diagnosis of acute aortic syndrome. Am J Med 2004;116:73-7. [Crossref] [PubMed]
- Burkhart HM, Gomez GA, Jacobson LE, et al. Fatal blunt aortic injuries: a review of 242 autopsy cases. J Trauma 2001;50:113-5. [Crossref] [PubMed]
- Steenburg SD, Ravenel JG, Ikonomidis JS. Blunt traumatic injury of the ascending aorta: multidetector CT findings in two cases. Emerg Radiol 2007;13:217-21. [Crossref] [PubMed]


