Diagnosis and interventions of vascular complications in lung transplant
Introduction
The incidence of lung transplant procedures in the US has progressively increased since the first human lung transplant was performed in 1963 (1,2). Lung transplantation is now an established treatment modality for a broad spectrum of end-stage pulmonary diseases (3). The most common indications for lung transplantation accounting for approximately 85% of all procedures are advanced chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF), cystic fibrosis (CF), emphysema due to alpha-1 antitrypsin deficiency, and pulmonary arterial hypertension (PAH). The remaining 15% comprise of end stage lung diseases including sarcoidosis, lymphangiomyomatosis and end stage histiocytosis. The types of lung transplants performed include single-lung transplant, bilateral sequential lung transplants, and heart-lung transplants.
Myriad of pulmonary complications can occur after lung transplantation that can be classified in the following categories: (I) primary graft dysfunction; (II) airway-related and vascular complications; (III) immediate postoperative complications; (IV) infections; (V) immunological complications (rejection); (VI) neoplasms; and (VII) iatrogenic complications (3). Incidence of various complications varies depending on the time line from the transplant surgery with an overall 42.8% risk of infection and a 10% risk of rejection. Approximately 19.9% of transplant recipients require reoperation (4). The management strategies have been more effective at reducing early complications compared to late, possibly due to improvement in surgical techniques and postoperative care. However, survival beyond the first year is primarily affected by chronic rejection and infections, and the incidence of these complications has not changed substantially in the past few decades.
According to the 2017 registry report by the International Society for Heart and Lung Transplantation (ISHLT), the median survival for all adult recipients is 6 years, with a better survival for bilateral lung transplant (BLT) recipients (5). Recipients with COPD have the best 1-year survival (6).
Vascular anastomotic complications following lung transplantation are associated with significant morbidity and mortality, with an incidence ranging from 1.8–5.2% (7,8). Prognosis depends on early diagnosis, type of complication, time elapsed after transplantation, clinical condition of the patient, and selection of the most appropriate therapy (9). We will review and discuss mainly the vascular complications and their management in this article.
Anastomotic surgical technique
Lung transplantation is a complex surgical procedure usually performed with clamps on the recipient pulmonary artery (PA) and left atrial (LA) cuff, with or without cardiopulmonary bypass (CPB). Vascular injury remains a concern secondary to engagement of the clamp which possibly compromises the integrity of the anastomosis. A “no clamp” technique has been described for vascular anastomosis in lung transplant which is feasible and safe (10). Advantages of the no clamp technique include reduction of warm ischemia and CPB time, and improvement of clinical outcomes. It may also reduce the frequency of post-lung transplantation atrial arrhythmias (10).
Both pulmonary arterial and venous anastomoses are created using a continuous suture technique. The PA is divided proximal to the first arterial branch on both the recipient and donor unless there is a size mismatch secondary to dilated arteries. The arterial anastomosis is more prone to narrowing and kinking when there is poor orientation, severe donor/recipient PA diameter mismatch, or excessive length. The pulmonary venous anastomosis utilizes a standard LA cuff technique in order to create a wider confluent anastomosis; hence, it is easier to orient the pulmonary venous anastomosis thereby reducing the likelihood of anastomotic suture stenosis or thrombus on the venous side.
Vascular complications
Anastomotic stenosis and pulmonary embolism are the most common pulmonary vascular complications following lung transplantation. The incidence of vascular anastomotic complications ranges from 1.8% to 15% (7,8) in different series of studies with a lower incidence of arterial complications (11).
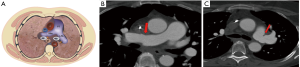
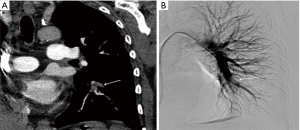
Anastomotic pulmonary arterial stenosis (PAS) was defined by Hausmann in 1992 as an anastomotic diameter of less than 75% compared to the neighboring vessels. It is a rare complication with a reported incidence of less than 2%. A mild degree of stenosis at the arterial anastomosis without hemodynamic significance is a normal finding after transplantation secondary to donor-receptor size discordance or secondary to the suture technique (Figure 1).
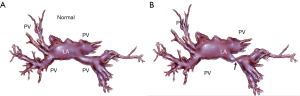
Pulmonary venous stenosis (PVS) is a rare complication that occurs usually in early postoperative period (first 48 h) after lung transplantation (Figure 2). If left untreated PVS may lead to venous thrombosis and transplant failure. The incidence of venous thrombosis is about 15% (11). The inferior pulmonary veins and particularly the left lower pulmonary vein is most commonly involved secondary to their anatomical position and predisposition to suture stenosis (12).
Incidence of pulmonary arterial embolism in patients following lung transplantation has been reported to be ranging from 1–8%. Aside from the transplant surgery itself, other risk factors for venous thrombosis and embolism (VTE) after lung transplant include hypercoagulable state, age, immobility, the presence of central venous catheters, antirejection medications, diabetes and post-transplant pneumonia. It is not uncommon for a lung transplant patient to have multiple of these risk factors placing them in a high VTE risk population.
Five types of vascular complications at the anastomosis have been described. Type 1 is described as kinking of the anastomosis due to excessive length of the donor and recipient segments, distortion of the anastomosis due to inadequate donor vessel length or hilar misalignment. Distortion or kinking of the anastomosis leads to anastomotic narrowing and thrombosis. Type 2 results from transposition of the donor vessel with respect to the recipient. Type 3 is a true stricture of the anastomosis secondary to overtightening or misalignment of the suture line. Type 4 is due to intraluminal obstruction secondary to thrombosis (4A) or dissection (4B). Type 5 refers to extra luminal mass effect associated with the use of omental pedicles (11-14). Conditions that make the vascular anastomosis technically more challenging include previous recipient lung surgery involving the hilum, fibrotic lung disease, female patients (possibly related to small thoracic cavity), small vascular structures, kyphoscoliosis, donor-recipient size mismatch, and lobar transplant (14-17).
Clinical presentation
Signs and symptoms that should raise suspicion for a vascular complication include persistent dry cough, dyspnea, pulmonary edema, unexplained hypoxia with ventilator dependence, and decline in gas exchange postoperatively, particularly if associated with pulmonary hypertension and hemodynamic compromise. Entities such as primary graft dysfunction, infection, acute rejection and myocardial dysfunction may present similarly and need to be excluded. Nevertheless, a high index of suspicion for vascular complications is warranted as the clinical features and imaging findings are often non-specific, especially if the symptoms have not improved despite being treated for the above mentioned common postoperative complications. Early identification of PAS is important as improved clinical outcomes have been reported following prompt treatment. Mean time to diagnosis of this complication is about 9 days. Unilateral vascular constriction may remain asymptomatic if there has been a bilateral lung transplant as compared to vascular obstruction in single lung transplant (7). Risk of pulmonary infarction is greatest in immediate post-operative period as there is no bronchial circulation available to provide perfusion due to surgical ligation. Compared to normal lungs, transplanted lungs have only a single source of blood supply and bronchial artery neovascularization takes multiple weeks (18). This means that during this period, the bronchi are supplied by poorly oxygenated blood from the pulmonary arteries in a retrograde fashion through the submucosal plexus at the level of the distal airways. Compromise of the PA during this period results in pulmonary infarction with high associated mortality (19).
Diagnostic modalities
Various diagnostic modalities are available for diagnosis of vascular complications including echocardiography, intra-operative ultrasound, CT and MRI. The individual role of these modalities depends on the timing of clinical presentation after surgery.
Intra-operative diagnosis
Echocardiography
Intraoperative anastomotic narrowing can be assessed by direct inspection, or intraoperative imaging with transthoracic echocardiography (TTE) or transesophageal echocardiography (TEE). The advantage of using this modality is that it is non-invasive and easy to operate but requires expertise especially for diagnosis of pulmonary venous thrombosis that can be challenging with post-operative altered anatomy (20). Identification and quantification of left inferior pulmonary venous anastomosis in particular may be difficult to visualize with TEE (16,17).
Intra-operative ultrasound
Felten et al. have recently advocated the use of intraoperative contact ultrasound (21). They compared intraoperative TEE and contact ultrasound and found that contact ultrasound produced better left pulmonary arterial recordings whereas TEE was more accurate for venous velocities.
Needle manometry line
An easy and convenient method that can be incorporated into routine practice is intraoperative direct measurement of pressure gradient across the anastomosis at a period of hemodynamic stability with a needle manometry line. This process allows immediate surgical correction in case of a high pressure gradient across the vascular anastomosis (7).
Post-operative diagnosis
Radiograph
Imaging with chest X-ray may show pulmonary edema, with nonspecific opacities and /or pleural effusion.
Ventilation-perfusion (V/Q) scan
A V/Q scan does not allow for intraoperative correction, and has a very limited role. In the post-operative period, A V/Q scan may show absent perfusion and ventilation in lung infarction secondary to pulmonary arterial occlusion and is useful in a setting where contrast is not administered for CT.
CT
Contrast enhanced CT (CECT) imaging is a readily available non-invasive modality that allows more accurate postoperative evaluation of both arterial and venous stenosis, especially with post-processing techniques that include multiplanar reformations (MPRs), volume rendering, and minimum- and maximum-intensity-projection images (22). It is important to direct the CT protocol beforehand as administration of IV contrast in the immediate post-operative period may have unwanted consequences and close collaboration and discussion with the transplant team is an important part of workflow in the management of these patients. For CT pulmonary angiography (CTPA), a volumetric scan is recommended on a multislice CT scanner using automatic tube current modulation and thinnest collimation available. Automated bolus tracking technique can be used to determine the scanning delay and approximate 75–100 mL of nonionic contrast is administered at a rate of 4 mL/s using a power injector. In addition to the assessment of the extent and degree of stenosis, CT also provides information about the lung parenchyma, distal vasculature, collateral vessel, airways, and pleural space. The 3D volume rendered angiographic reconstructions are useful for pretreatment planning. Normally on CECT, vascular sites of anastomosis are visualized as 1–2 mm folds on the walls of the blood vessels without a significant reduction in the diameter (Figure 1) (23). Significant change in caliber of the pulmonary vessel is an important indicator of stenosis (Figures 3,4). In cases of pulmonary embolism, intraluminal filling defects and abrupt cutoff of vessels are important indicators of acute pulmonary embolism. Multiplanar reconstructions and maximum intensity projection (MIP) images aid in differentiating mucus-filled airways or lymph nodes from pulmonary emboli. The multiplanar capability is also useful in determining success of treatment on follow up scans.
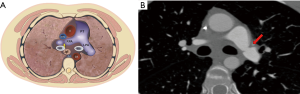
MR angiography (MRA)
MRA is an important alternative to CTPA is an important non-ionizing alternative to CTPA in younger patients where radiation remains a concern. Advancement in MRI technology including view sharing, parallel imaging and time-resolved MRA, have shortened the acquisition times and have led to increased use of MRA for the primary diagnosis of PE as compared to longer acquisition times, limited volumetric coverage and lower spatial resolution than CTPA in the past years.
The most important sequences in a pulmonary CE-MRA protocol are those acquired after the administration of an intravenous (i.v.) contrast agent and acquired using a rapid three-dimensional (3D) spoiled gradient-echo sequence. The vascular phases of pulmonary CE-MRA, enhance the pulmonary arterial system to facilitate identification of intraluminal filling defects. Non-contrast MRA using bright blood balanced steady-state free-precession (bSSFP) sequence are more widely used for assessing pulmonary arteries. Non-contrast bSSFP examinations perform comparably with CE-MRA when evaluating the central and lobar pulmonary arteries (24). A post-contrast T1 weighted spoiled gradient recalled echo (SGRE) acquisition with fat saturation can be of additional advantage in assessment for other pathology in the mediastinum and chest wall.
Dedicated perfusion imaging can also be accomplished using dynamic contrast-enhanced MRI sequences that can appraise qualitative and quantitative perfusion of the lung parenchyma and add to the functional significance of a perfusion defect (25).
Pulmonary angiography (PA)
Although routinely used prior to 1995, PA is now reserved for instances in which a catheter-based intervention may be required and remains the most commonly used confirmatory modality. PA is selected with a pigtail or an angled pigtail catheter via common femoral or internal jugular vein approach. Comprehensive evaluation includes pulmonary arterial pressure measurements followed by pulmonary angiogram in multiple projections.
PA demonstrates filling defects consistent with thrombus, either at the level of the anastomosis or peripherally. In cases suspected or known to have large pulmonary embolism or severe PAH, an initial hand injection of contrast material is the preferred option.
Arterial anastomotic stenosis is well characterized on the pre-procedural CT scan and helps in planning for subsequent intervention. Findings of anastomotic stenosis on angiogram include change in vessel caliber to complete stenosis. Pressure measurement with a gradient across the area of stenosis is useful, especially when the findings of stenosis are equivocal. Additionally, pressure gradient measurement is an important parameter to assess the response during therapy. Based on the vessel caliber immediately central to the stenotic area, balloon dilatation and angioplasty are planned. Evaluation of venous anastomosis requires delayed imaging to result in opacification of the pulmonary veins whereas interventions at this location require trans-septal access. It is very important to weight the treatment benefits with the risk of rupturing the friable anastomosis, especially in the immediate and early post-operative periods.
Treatment
Management of complications
Immediate
Mild anastomotic narrowing may be treated conservatively, particularly in the setting of bilateral lung transplant. However a significant stenosis at the arterial or venous anastomosis that compromises the graft and results in clinical deterioration requires immediate intervention. Open interventions to salvage the transplanted lung carry a significant risk and limited reports are available on success rates on open repair of PAS. Ischemic reperfusion injury to the donor organ secondary to vascular clamping during the revision surgery is associated with increased postoperative morbidity, and complications (15,26,27).
Significant anastomotic narrowing can also be treated by catheter based intervention or surgical revision. Endovascular versus surgical treatment is guided by the time that has elapsed after the anastomosis to diagnosis, anatomy of the lesion, clinical condition of the patient and ability to tolerate another intervention in addition to available expertise and experience.
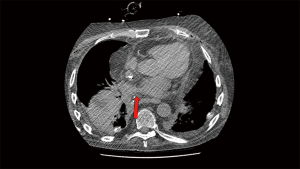
For vascular anastomotic thrombosis, anticoagulation with heparin and close clinical monitoring should be the initial management. Severe thrombosis with potential for graft infarction may necessitate surgical intervention, but endovascular interventions are also an option with various thrombectomy devices and techniques (chemical, mechanical, aspiration, etc.) available and may be used based on individual preference and expertise. Catheter directed thrombolysis (CTD) with tissue plasminogen (tPA) can be performed with catheters across the area of thromboembolism with close monitoring of vitals and coagulation parameters. CTD provides maximum local lytic dose and decreases the systemic lytic dose, thereby offering the advantage of increased lytic efficacy and decreased bleeding risk compared to systemic thrombolysis (28).
CTD treatment in lung transplant patients is similar to treatment of pulmonary embolism in non-transplant patients except for careful consideration of friable anastomosis, and a relative contraindication in the immediate postoperative period (Figures 5,6). Despite this relative contraindication, the risk of bleeding must be weighed against the risk of graft loss and death.
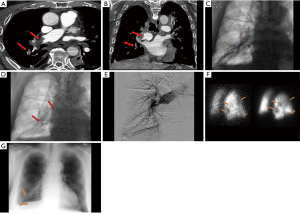
Intermediate
In the first 2 weeks after transplantation, it remains debatable as to the preferred approach for treatment of vascular anastomotic narrowing and thrombosis. A surgical approach is generally favored as the integrity of the anastomosis is friable given the short interval time from the time of surgery. Surgery may also be necessary in cases when there is inversion of the donor vessel with respect to the recipient. The transplanted lung is cooled on CPB and cold pulmoplegic solution is utilized to prevent warm ischemia and infarction. Successful treatment is attributed to stabilization and preventing twisting of the anastomosis. In the scenario where the anatomy is favorable and expertise is available, an endovascular treatment with careful balloon dilatation and stenting is an option with careful attention to friable post-operative vasculature, and risk of complications including stent migration, thrombosis, restenosis and embolization.
Delayed
For cases diagnosed in the delayed (several weeks) postoperative period, multiple groups have reported success with endovascular techniques and interventions (29-32). Excellent functional and anatomic outcomes with low mortality and morbidity rates after percutaneous angioplasty with or without vascular endoprosthesis were reported for severe anastomotic stenosis of the PA (30,33,34). Most pulmonary arterial lesions require stent placement due to the elasticity of the lesions, although angioplasty without stent placement is also an alternative option (9). A second stent may be deployed partially inside the first one to cover for insufficient length over the lesion with good short- term functional and anatomic outcomes. For persistent or recurrent stenosis, deployment of a balloon-mounted stent is preferred over a self-expanding stent. No specific guidelines exist regarding the follow up after endovascular intervention. Short term anticoagulation and/or antiplatelet therapy is the cornerstone for maintaining patency of the stents. Follow up imaging utilizing a CECT or angiogram can be obtained at short term intervals or depending on the clinical symptoms. Nevertheless, presence of an endovascular stent may make repeat lung transplantation cumbersome. Extensive dissection towards the hilum is required to ensure that clamp can be placed beyond the stent and allow safe removal of the stent.
In patients with complete venous obstruction, repeat surgery, re-transplantation or lobectomy for double lung transplantation may be required. However, if the condition of the patient is stable and a favorable anatomy is identified, angioplasty with dilation and endovascular stent implant has been shown to be successful (15).
Conclusions
Vascular complications after lung transplantation are rare but can result in high mortality and morbidity if left untreated. The arterial anastomosis is more prone to complications of stenosis and thrombosis compared to venous anastomosis due to surgical technique and pathophysiology. Since the signs and symptoms are non-specific, a high index of suspicion is needed to prevent a missed diagnosis. Diagnosis is primarily via echocardiogram or CTA imaging. CTA has additional benefits of advanced post-processing capabilities and being able to image the lung parenchyma. Surgical and endovascular interventional techniques have both demonstrated successful salvage of the organ and the patient in treating post lung transplant complications. Endovascular stent placement for transplant related arterial stenosis has a low mortality and morbidity with successful functional and anatomic outcomes in the short term.
PE is problematic due to compromised bronchial circulation with possible infarction and graft loss and is associated with a high mortality. Catheter directed lysis is an alternative to embolectomy/surgical revision with the need to weigh in bleeding risks and friable anastomosis compared to re-operative risks. Both surgery/re-transplantation and intervention techniques on vascular structures after lung transplantation require careful multidisciplinary evaluation and high procedural expertise.
Acknowledgements
Erin Moore, Medical Illustrator Department of Radiology, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hardy JD, Webb WR, Dalton ML Jr, et al. Lung homotransplantation in man. JAMA 1963;186:1065-74. [Crossref] [PubMed]
- Cooper JD, Patterson GA, Grossman R, et al. Double-lung transplant for advanced chronic obstructive lung disease. Am Rev Respir Dis 1989;139:303-7. [Crossref] [PubMed]
- Tejwani V, Panchabhai TS, Kotloff RM, et al. Complications of Lung Transplantation: A Roentgenographic Perspective. Chest 2016;149:1535-45. [Crossref] [PubMed]
- Kilic A, George TJ, Beaty CA, et al. The effect of center volume on the incidence of postoperative complications and their impact on survival after lung transplantation. J Thorac Cardiovasc Surg 2012;144:1502-8; discussion 1508-9. [Crossref] [PubMed]
- Meyer DM, Edwards LB, Torres F, et al. Impact of recipient age and procedure type on survival after lung transplantation for pulmonary fibrosis. Ann Thorac Surg 2005;79:950-7; discussion 957-8. [Crossref] [PubMed]
- Chambers DC, Yusen RD, Cherikh WS, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Lung And Heart-Lung Transplantation Report-2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant 2017;36:1047-59. [Crossref] [PubMed]
- Siddique A, Bose AK, Ozalp F, et al. Vascular anastomotic complications in lung transplantation: a single institution's experience. Interact Cardiovasc Thorac Surg 2013;17:625-31. [Crossref] [PubMed]
- Griffith BP, Magee MJ, Gonzalez IF, et al. Anastomotic pitfalls in lung transplantation. J Thorac Cardiovasc Surg 1994;107:743-53; discussion 753-4. [PubMed]
- Shoji T, Hanaoka N, Wada H, et al. Balloon angioplasty for pulmonary artery stenosis after lung transplantation. Eur J Cardiothorac Surg 2008;34:693-4. [Crossref] [PubMed]
- Mohite PN, Garcia-Saez D, Sabashnikov A, et al. No-clamp technique for pulmonary artery and venous anastomoses in lung transplantation. J Heart Lung Transplant 2014;33:1133-8. [Crossref] [PubMed]
- Schulman LL, Anandarangam T, Leibowitz DW, et al. Four-year prospective study of pulmonary venous thrombosis after lung transplantation. J Am Soc Echocardiogr 2001;14:806-12. [Crossref] [PubMed]
- Malden ES, Kaiser LR, Gutierrez FR. Pulmonary vein obstruction following single lung transplantation. Chest 1992;102:645-7. [Crossref] [PubMed]
- González-Fernández C, Gonzalez-Castro A, Rodriguez-Borregan JC, et al. Pulmonary venous obstruction after lung transplantation. Diagnostic advantages of transesophageal echocardiography. Clin Transplant 2009;23:975-80. [Crossref] [PubMed]
- Sakamaki Y, Minami M, Ohta M, et al. Pulmonary artery dissection complicating lung transplantation for primary pulmonary hypertension. Ann Thorac Surg 2006;81:360-2. [Crossref] [PubMed]
- Clark SC, Levine AJ, Hasan A, et al. Vascular complications of lung transplantation. Ann Thorac Surg 1996;61:1079-82. [Crossref] [PubMed]
- Michel-Cherqui M, Brusset A, Liu N, et al. Intraoperative transesophageal echocardiographic assessment of vascular anastomoses in lung transplantation. A report on 18 cases. Chest 1997;111:1229-35. [Crossref] [PubMed]
- Miyaji K, Nakamura K, Maruo T, et al. Effect of a kink in unilateral pulmonary artery anastomosis on velocities of blood flow through bilateral pulmonary vein anastomoses in living-donor lobar lung transplantation. J Am Soc Echocardiogr 2004;17:998-9. [Crossref] [PubMed]
- Hyytinen TA, Heikkila LJ, Verkkala KA, et al. Bronchial artery revascularization improves tracheal anastomotic healing after lung transplantation. Scand Cardiovasc J 2000;34:213-8. [Crossref] [PubMed]
- Santacruz JF, Mehta AC. Airway complications and management after lung transplantation: ischemia, dehiscence, and stenosis. Proc Am Thorac Soc 2009;6:79-93. [Crossref] [PubMed]
- de la Torre M, Fernandez R, Fieira E, et al. Postoperative surgical complications after lung transplantation. Rev Port Pneumol (2006) 2015;21:36-40. [PubMed]
- Felten ML, Michel-Cherqui M, Sage E, et al. Transesophageal and contact ultrasound echographic assessments of pulmonary vessels in bilateral lung transplantation. Ann Thorac Surg 2012;93:1094-100. [Crossref] [PubMed]
- Gill RR, Poh AC, Camp PC, et al. MDCT evaluation of central airway and vascular complications of lung transplantation. AJR Am J Roentgenol 2008;191:1046-56. [Crossref] [PubMed]
- Hemmert C, Ohana M, Jeung MY, et al. Imaging of lung transplant complications. Diagn Interv Imaging 2014;95:399-409. [Crossref] [PubMed]
- Benson DG, Schiebler ML, Repplinger MD, et al. Contrast-enhanced pulmonary MRA for the primary diagnosis of pulmonary embolism: current state of the art and future directions. Br J Radiol 2017;90. [Crossref] [PubMed]
- Ingrisch M, Maxien D, Meinel FG, et al. Detection of pulmonary embolism with free-breathing dynamic contrast-enhanced MRI. J Magn Reson Imaging 2016;43:887-93. [Crossref] [PubMed]
- Soriano CM, Gaine SP, Conte JV, et al. Anastomotic pulmonary hypertension after lung transplantation for primary pulmonary hypertension: report of surgical correction. Chest 1999;116:564-6. [Crossref] [PubMed]
- Anaya-Ayala JE, Loebe M, Davies MG. Endovascular management of early lung transplant-related anastomotic pulmonary artery stenosis. J Vasc Interv Radiol 2015;26:878-82. [Crossref] [PubMed]
- Bousamra M 2nd, Mewissen MW, Batter J, et al. Pulmonary artery thrombolysis and stenting after a bilateral sequential lung transplantation. J Heart Lung Transplant 1997;16:678-80. [PubMed]
- Banerjee SK, Santhanakrishnan K, Shapiro L, et al. Successful stenting of anastomotic stenosis of the left pulmonary artery after single lung transplantation. Eur Respir Rev 2011;20:59-62. [Crossref] [PubMed]
- Berger H, Steiner W, Schmidt D, et al. Stent-angioplasty of an anastomotic stenosis of the pulmonary artery after lung transplantation. Eur J Cardiothorac Surg 1994;8:103-5. [Crossref] [PubMed]
- Ferretti G, Boutelant M, Thony F, et al. Successful stenting of a pulmonary arterial stenosis after a single lung transplant. Thorax 1995;50:1011-2; discussion 1016-7. [Crossref] [PubMed]
- Waurick PE, Kleber FX, Ewert R, et al. Pulmonary artery stenosis 5 years after single lung transplantation in primary pulmonary hypertension. J Heart Lung Transplant 1999;18:1243-5. [Crossref] [PubMed]
- Grubstein A, Atar E, Litvin S, et al. Angioplasty using covered stents in five patients with symptomatic pulmonary artery stenosis after single-lung transplantation. Cardiovasc Intervent Radiol 2014;37:686-90. [Crossref] [PubMed]
- Zimmermann GS, Reithmann C, Strauss T, et al. Successful angioplasty and stent treatment of pulmonary vein stenosis after single-lung transplantation. J Heart Lung Transplant 2009;28:194-8. [Crossref] [PubMed]


