Pericarditis-complicated takotsubo cardiomyopathy in a patient with rheumatoid arthritis
Introduction
Takotsubo cardiomyopathy (TTC) is well-known to be a stress-induced cardiomyopathy. Several reports have described concomitant TTC and pericarditis, but the pathogenesis of the association between TTC and pericarditis remains unclear (1-3). On the other hand, an association of TTC with autoimmune disorders has been reported in the literature (4-6). Here, we present a case of TTC, complicated by pericarditis, in a patient with rheumatoid arthritis (RA).
Case presentation
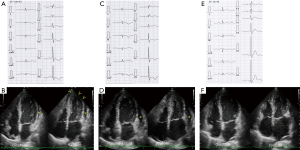
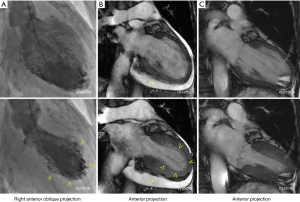
A 64-year-old woman was admitted to our hospital with acute-onset chest pain and worsening shortness of breath that began 3 days prior to admission. She had a history of methotrexate-controlled RA, diagnosed 8 years earlier. On physical examination, her blood pressure was 103/75 mmHg, her pulse rate was 86 beats per minute and body temperature was 36.8 °C. Jugular venous distension was noted, and a grade 2, low-pitched, systolic murmur was heard between the apex of the heart and the sternum. An electrocardiogram (ECG) showed incomplete right bundle-branch block, elevated ST-segments in leads II, III and aVF, and inverted T-waves in leads V3–6, with a prolonged QTc interval of 0.45 s (Figure 1). Echocardiography demonstrated left ventricular apex akinesis and moderate pericardial effusion (Figure 1B). Laboratory tests revealed a positive troponin-T test, a normal creatine kinase level, and a slightly elevated serum C-reactive protein level (0.94 mg/dL; normal value, <0.5 mg/dL). Other results are shown in Table 1. Coronary angiography indicated the absence of obstructive coronary artery disease, and left ventriculography demonstrated apical ballooning (akinesis of the apical ventricle with hypercontractility of the base) (Figure 2A). Cardiac magnetic resonance imaging (CMR) also demonstrated apical ballooning of the left ventricle, and moderate pericardial effusion (Figure 2B). No late gadolinium enhancement (LGE) of the myocardium and the pericardium was detected. Computed tomography showed that the Hounsfield unit value for the pericardial effusion was 28, suggesting an exudative, non-hemorrhagic effusion (7). The patient was diagnosed with pericarditis-complicated TTC, and treated conservatively, without corticosteroids or anticoagulants. On the other hand, the patient had not an antecedent viral syndrome with fever, and malaise. Furthermore, the typical wall motion abnormalities of TTC on left ventriculography was demonstrated, and LGE on CMR was absent. Accordingly, acute myocarditis was excluded (8). Five days later, an ECG showed resolution of the ST-segment elevations in leads II, III, and aVF, but persistence of the inverted T-waves in leads V3–6 (Figure 1C). Repeated echocardiography showed recovery of the left ventricular wall motion, but a slight increase in the pericardial effusion (Figure 1D). The patient’s serum C-reactive protein level had decreased to 0.53 mg/dL. Five months later, the T-wave inversions had returned to normal (Figure 1E) and the pericardial effusion had resolved (Figure 1F). Ten months later, CMR demonstrated normal left ventricular wall motion and resolution of the pericardial effusion (Figure 2C). The patient has been symptom-free for a year.
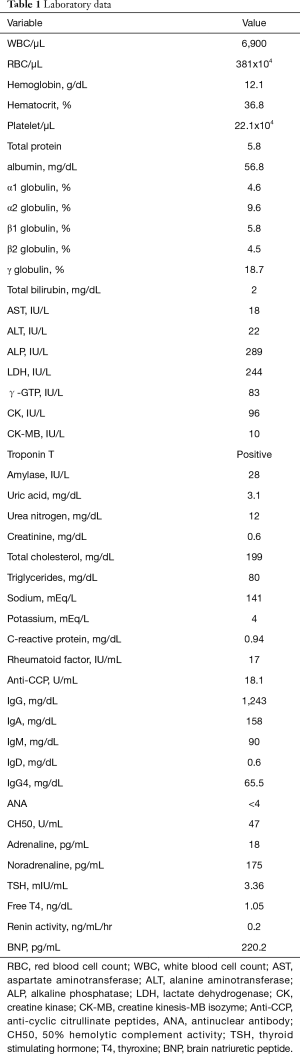
Full table
Discussion
Associations have been reported between TTC and several autoimmune disorders (i.e., Hashimoto thyroiditis, Dressler’s syndrome, autoimmune polyendocrine syndrome, and RA) (4-6). The postulated pathogenic mechanisms include increased susceptibility to catecholamine, increased sympathetic nerve activity, and microvasculature dysfunction in the setting of systemic inflammation (3,4). There are several reports of TTC in which the disorder appeared to be caused by biopsy-proven inflammation (9-12). On the other hand, RA is occasionally complicated by pericarditis (13). In our patient, her pericardial effusion developed rapidly and resolved over a short period of time, without corticosteroid therapy. Therefore, we consider the pericardial effusion to have been mainly caused by the TTC. In our patient, we presume that the coexistence of TTC and RA might have played a role in the etiology of a significant accumulation of pericardial fluid. Further study is needed to investigate a relationship between these three conditions (TTC, pericarditis, and RA). This patient should be closely followed for pericarditis recurrence and for the development of constrictive pericarditis, cardiac tamponade, and heart failure.
In summary, we reported a case of pericarditis-complicated TTC in a patient with RA. We consider that the pericardial effusion was caused by TTC, and that the coexistence of RA might have played a role in the etiology of the significant pericardial fluid accumulation. Further investigations are needed in this regard.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Guevara R, Aguinaga-Meza M, Hazin MI, et al. Takotsubo cardiomyopathy complicated with acute pericarditis and cardiogenic shock. J Natl Med Assoc 2007;99:281-3. [PubMed]
- Maruyama T, Hanaoka T, Nakajima H. Acute pericarditis in the recovery phase of transient left ventricular apical ballooning syndrome (takotsubo cardiomyopathy). Intern Med 2007;46:1857-60. [Crossref] [PubMed]
- Eitel I, Lucke C, Grothoff M, et al. Inflammation in takotsubo cardiomyopathy: insights from cardiovascular magnetic resonance imaging. Eur Radiol 2010;20:422-31. [Crossref] [PubMed]
- Ugurlucan M, Zorman Y, Ates G, et al. Takotsubo cardiomyopathy in a patient with multiple autoimmune disorders and hyperthyroidism. Res Cardiovasc Med 2013;2:145-8. [Crossref] [PubMed]
- Gasparyan AY, Cocco G, Pandolfi S. Cardiac complications in rheumatoid arthritis in the absence of occlusive coronary pathology. Rheumatol Int 2012;32:461-4. [Crossref] [PubMed]
- Lim T, Murakami H, Hayashi K, et al. Takotsubo cardiomyopathy associated with autoimmune polyendocrine syndrome II. J Cardiol 2009;53:306-10. [Crossref] [PubMed]
- Çetin MS, Özcan Çetin EH, Özdemir M, et al. Effectiveness of computed tomography attenuation values in characterization of pericardial effusion. Anatol J Cardiol 2017;17:322-7. [PubMed]
- Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA 2011;306:277-86. [PubMed]
- Komamura K, Fukui M, Iwasaku T, et al. Takotsubo cardiomyopathy: Pathophysiology, diagnosis and treatment. World J Cardiol 2014;6:602-9. [Crossref] [PubMed]
- Kawai S, Shimada T. Inflammation in takotsubo cardiomyopathy? Inquiry from "Guidelines for Diagnosis and Treatment of Myocarditis (JCS 2009)". J Cardiol 2014;63:247-9. [Crossref] [PubMed]
- Fisher P, Sidhu R, Behuria S, et al. Pericardial effusion in the setting of Takotsubo cardiomyopathy. J Integr Cardiol 2015;1:218-9.
- Bybee KA, Kara T, Prasad A, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med 2004;141:858-65. [Crossref] [PubMed]
- Calick A, Bishop R. Pericardial Effusion in Rheumatoid Arthritis. Chest 1973;64:778-9. [Crossref] [PubMed]


