
Cardioprotection by very mild hypothermia in mice
Introduction
Hypothermia after cardiac arrest is known to improve neurologic outcome. Additionally, it exhibits cardioprotection in animal models of acute coronary infarction (1). However, data of hypothermia in patients are contradictory (2-4). Improvement of neurological outcome, even delayed hypothermia after return of successful resuscitation is beneficial (5). In contrast, delayed target temperature hypothermia at the time of reperfusion failed to reduce infarct size (IS) in models of myocardial ischemia (6). Additionally, the optimal target temperature after myocardial infarction (MI) is not clear. By now, most studies applied a regimen of mild hypothermia (~33 °C) (2,3). On the other hand, even mild hypothermia in conscious patients causes psychical and physical discomfort. Data from animal studies showed a strong correlation between body temperature and cardioprotection with a reduction of IS up to 20% per 1 °C. Therefore, a regimen of very mild hypothermia (34–36 °C) starting preclinical might be sufficient to reduce IS without causing fear, resist and shivering in patients. Hence, in this pilot study we aimed to analyze the impact of very mild hypothermia on IS in mice.
Methods
Animals and myocardial ischemia/reperfusion protocol
Totally, 12±2 weeks old C57BL/6 wilt type mice were used for experiments (n=12). Mice were purchased from Janvier Labs (Saint-Berthevin, France) and were kept on standard rodent chow. Mice were anesthetized with isoflurane and intubated. Anesthesia was maintained with 2 Vol% isoflurane. Left anterior descending (LAD) artery was ligated. Occurrence of characteristic electrocardiographic ST-elevation was used as control of successful ligation. In hypothermic mice, external cooling was conducted. Ice packs were applied to achieve external cooling. Body temperature was continuously controlled by a rodent rectal probe thermometer. Warming plate was used in control mice to reduce loss of temperature during open chest ischemia reperfusion surgery. Ischemia was maintained for 30 or 60 minutes respectively. Ligation was resolved and regression of ST elevation was monitored. Hypothermic mice were rewarmed. After 24 hours of reperfusion, mice were sacrificed and the heart was excised. The study was approved by the German Animal Care and Use Committee (LANUV NRW). Care and handling was according to the German Animal Care and Use guidelines.
Myocardial IS measurement
After excision, re-ligation of the LAD at the same location was conducted and hearts were perfused with Evans blue dye. This distinguished the area at risk from non-ischemic myocardium. Hears storage at −20 °C for 1 hour, hearts were sectioned followed by 2,3,5-triphenyltetrazolium chloride incubation. Computer assisted planimetry was used to measure the IS and the area at risk. MI was expressed as IS/area at risk (AAR).
Statistical analyses
GraphPad Prism statistical software (GraphPad Software Inc., San Diego, USA) was used for statistical analysis. Data are mean ± standard deviation (SD). P<0.05 was considered significant. Student’s t-test was used to analyze data.
Results and discussion
As shown in Figure 1, temperature at baseline did not differ between hypothermic mice and control mice (35.7±0.2 vs. 35.9±0.4 °C; no significant) Hypothermia was achieved within 5 minutes of ischemia (34.5±0.5 °C) and maintained during the time course of ischemia. IS/AAR after 30 minutes as well as 60 minutes ischemia was smaller in mice undergoing very mild hypothermia as compared to control mice (30 min ischemia: 22±4% vs. 45±11%; P=0.018; n=6; 60 min ischemia: 28±2% vs. 67±7%; P<0.001; n=6) (Figure 2).
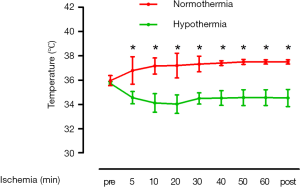
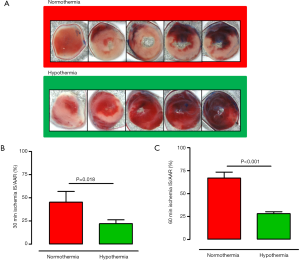
The major finding of this study was that very mild hypothermia with a temperature of 34–36 °C is sufficient to reduce IS in mice undergoing myocardial ischemia. This differed from clinical studies that aimed to achieve a body temperature of 32–34 °C.
Hypothermia is already state of the art in neuroprotection in patients after cardiac arrest (7). Additionally, it is a very promising option in cardioprotection in patients with acute MI. However, there are still many open questions regarding the optimal target temperature and optimal setting of hypothermia in patients with acute MI. However, the results of clinical studies using hypothermia to minimize IS in patients with acute MI were inconsistent. The VELOCITY study (32–34 °C target temperature) investigated rapid induction of hypothermia by using a peritoneal catheter. Hypothermia was induced prior to angiography. IS did not differ between hypothermia and control patients but major adverse cardiac events (MACE) were more frequent in the hypothermia group. Especially stent thrombosis contributed to MACE in patients undergoing hypothermia (4). This might be due to hypothermia induced affection of platelet reactivity and impairment of absorption and metabolization of antiplatelet drugs (8). The CHILL-MI trial induced hypothermia prior to coronary angiography in patients with MI by cold saline infusion and endovascular cooling. In this study a fixed protocol of cooling was applied without adjustment according to individual body temperature. Only patients with symptom onset to percutaneous coronary intervention (PCI) time below 4 hours and anterior ST-elevation MI had reduced IS as compared to control patients (3). Similar to the CHILL-MI trial, the Cool-MI and ICE-IT studies reported reduction in IS only in patients with anterior MI (9,10). Only the Rapid MI-ICE pilot trial was able to demonstrate reduction of IS in MI patients independently of localization of infarction. Remarkably, this pilot trial was the only one to sufficiently achieve target temperature by cold saline infusion and endovascular cooling without delaying time to intervention (2). Therefore, the key factors seem to be achieving hypothermia quickly without affecting time to PCI. However, hypothermia in conscious patients may cause fear and shivering. Additionally, platelet reactivity as well as pharmacokinetics and pharmacodynamic response to antiplatelet medication are affected by hypothermia (8). Therefore, the optimum seems to be only a very mild hypothermia which can be easily and rapidly induced without causing side effects. In this study, we were able to demonstrate that very mild hypothermia with a temperature between 34–36 °C is sufficient to reduce IS in mice undergoing ischemia reperfusion. The exact mechanism of reduced IS by hypothermia is not clear. It is known, that hypothermia affects cell death by modulation of reactive oxygen species, ATP metabolism, inflammation and apoptosis (11). On the other hand, hypothermia might affect haemostasis and platelet reactivity as mentioned above. This might have led to the increased rate of stent thrombosis in clinical trials. This underlines the significance of the present analysis that even very mild hypothermia already improves IS after AMI. This finding could be a new approach to improve outcome of acute MI by inducing very mild hypothermia. The safety and efficacy of this approach has to be investigated in patients with MI.
This study had different limitations. The sample size of mice was very small. However, results were significant. This underlines the effect size of this observation. No outliers were removed.
In conclusion, very mild hypothermia reduced IS by half in mice undergoing myocardial ischemia. Clinical trials are needed to assess the safety and efficacy of this approach in patients with acute MI.
Acknowledgements
We thank Stefanie Becher for experimental support.
Funding: Part of this work was supported by the Forschungskommission of the Medical Faculty of the Heinrich Heine University (No. 16-2014 to A.P.; No. 46-2016 to L.D.).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the German Animal Care and Use Committee (LANUV NRW). Care and handling was according to the German Animal Care and Use guidelines. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
References
- Hale SL, Dave RH, Kloner RA. Regional hypothermia reduces myocardial necrosis even when instituted after the onset of ischemia. Basic Res Cardiol 1997;92:351-7. [Crossref] [PubMed]
- Götberg M, Olivecrona GK, Koul S, et al. A pilot study of rapid cooling by cold saline and endovascular cooling before reperfusion in patients with ST-elevation myocardial infarction. Circ Cardiovasc Interv 2010;3:400-7. [Crossref] [PubMed]
- Erlinge D, Gotberg M, Lang I, et al. Rapid endovascular catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction. The CHILL-MI trial: a randomized controlled study of the use of central venous catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction. J Am Coll Cardiol 2014;63:1857-65. [Crossref] [PubMed]
- Nichol G, Strickland W, Shavelle D, et al. Prospective, multicenter, randomized, controlled pilot trial of peritoneal hypothermia in patients with ST-segment- elevation myocardial infarction. Circ Cardiovasc Interv 2015;8:e001965. [Crossref] [PubMed]
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002;346:549-56. [Crossref]
- Kang IS, Fumiaki I, Pyun WB. Therapeutic Hypothermia for Cardioprotection in Acute Myocardial Infarction. Yonsei Med J 2016;57:291-7. [Crossref] [PubMed]
- Nolan JP, Soar J, Cariou A, et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines for Post-resuscitation Care 2015: Section 5 of the European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation 2015;95:202-22. [Crossref] [PubMed]
- Ibrahim K, Christoph M, Schmeinck S, et al. High rates of prasugrel and ticagrelor non-responder in patients treated with therapeutic hypothermia after cardiac arrest. Resuscitation 2014;85:649-56. [Crossref] [PubMed]
- O’Neill WW, Dixon SR. The year in interventional cardiology. J Am Coll Cardiol 2004;43:875-90. [Crossref] [PubMed]
- O’Neill WW, Dixon SR, Grines CL. The year in interventional cardiology. J Am Coll Cardiol 2005;45:1117-34. [Crossref] [PubMed]
- Dae MW. Hypothermia and percutaneous coronary intervention during acute myocardial infarction. Interventional Cardiology 2012;4:235-43. [Crossref]


