
Emerging clinical applications of strain imaging and three-dimensional echocardiography for the assessment of ventricular function in adult congenital heart disease
Introduction
Two-dimensional (2D) transthoracic echocardiography (TTE) is the most widely used imaging modality for the assessment of cardiac anatomy and ventricular performance in patients with congenital heart disease (CHD) (1-6). It not only provides the clinician with the essential information to assure optimal patient management and planning for cardiac surgery and interventions, but it is also a widely available, feasible, safe and cost-effective modality for gathering details on the cardiovascular system (1-6). Moreover, 2D echocardiography combined with Doppler techniques and eventually contrast results in CHD to be diagnosed with a high degree of accuracy and reproducibility (1,7,8).
An important development in the standardization of multimodality imaging application in adult patients with CHD (ACHD) is the publication of several imaging guidelines from national and international organizations (9-12). Most of these guidelines provide an in-depth overview of the hemodynamic consequences of simple and complex CHD. Moreover, they discuss the strengths and weaknesses of different imaging modalities in a specific type of CHD, and describe useful recommendations on how serial monitoring for late complications is best provided for these patients (9-12). Thus, these guidelines help the cardiac team to assure that sufficient information on anatomic considerations and function is gathered to guide patient management and to provide the best possible care for these patients.
Heart failure due to myocardial dysfunction is frequently encountered in ACHD patients (13). It is a leading cause of death in ACHD, and is associated with high morbidity and decreased quality of life (14,15). Particularly patients with tetralogy of Fallot (TOF), a systemic RV [congenitally corrected transposition of the great arteries (ccTGA), D-TGA following atrial switch operation], or a Fontan circulation are at increased risk for developing HF (13-15). Therefore, long-term serial assessment of ventricular function is mandatory in the ACHD population. However, functional assessment of the right ventricle (RV) or left ventricle (LV) using conventional echocardiographic techniques may be challenging due to the complex cardiac geometry and presence of wall motion abnormalities (1-6). Standard echocardiographic functional parameters obtained with M-mode or volumetric methods rely on specific geometrical assumptions, which do not apply to volume and pressure overloaded (single) ventricles in CHD (1-6). As a result, qualitative echocardiographic grading of ventricular size and function is frequently being used in daily practice. The interobserver agreement for qualitative grading of ventricular function by echocardiography has been found to be modest for LV morphology and weak for RV morphology. Whereas, volume measurements of functional single ventricles by 2D-echocardiography underestimate cardiac magnetic resonance (CMR) measurements of volume (16).
Newer techniques, such as speckle tracking echocardiography (STE) and three-dimensional (3D) echocardiography may allow for detection of early myocardial dysfunction, before overt heart failure develops, and for better surveillance and risk stratification of ACHD patients at high risk for adverse events (4,17-20). Indeed, recent studies have demonstrated that these imaging modalities obtain reproducible and accurate quantitative measures of systolic function, ventricular size, and volume in CHD (4,17-20). The clinical applications of these imaging modalities are expanding quickly.
Therefore, in this review we discuss recent advances in the clinical application of deformation imaging and 3D-echocardiography in ACHD. We will focus on novel pathophysiological cardiac mechanisms in CHD, and the potential role of these newer echocardiographic techniques in clinical decision-making and patient care. The advances are highlighted in complex CHD, including TOF, systemic RV and Fontan.
Strain and strain rate
The physics and technical background of strain imaging and 3D echocardiography have been reviewed in detail elsewhere (12,20-22), and we will only summarize key concepts relevant for clinical application of these techniques. In brief, strain and strain rate can be obtained by tissue Doppler imaging (TDI) and STE (23). STE has largely replaced TDI as the preferred technique for measuring parameters of myocardial deformation because of the angle-independency and better reproducibility of STE obtained measurements (17,18). STE is based on the frame-by-frame tracking of specific gray-scaled areas in the myocardial tissue, commonly referred to as ‘speckles’, in all directions of the imaging plane using dedicated commercially available software (17,18,21,22). It is a validated and feasible echocardiographic method for the assessment of diastolic and systolic function in both children and adults with CHD.
Myocardial deformation is expressed as strain, which represents the fractional or percent change of a region of interest from its original dimension (change in length between two points) (21,22). Strain can be obtained in all 3 different directions (longitudinal, radial, and circumferential), and is physiologically linked to the complex myocardial fiber architecture of the human heart (24,25). Of importance, strain is considered a relative load-independent measure of myocardial function (contraction and relaxation), whereas strain rate represents the velocity of deformation (17,18). It should be noted, however, that our current physiological understanding on the meaning of strain and strain rate measurements in the complexity of CHD hemodynamics is still limited (26).
Left ventricular rotational mechanics
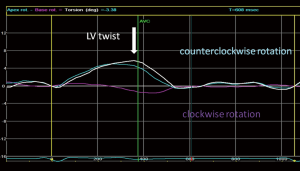
In addition to strain and strain rate measurements, STE is a well-studied tool to assess the rotational mechanisms of the LV (24,25). The oblique orientation of the subendocardial (right-handed) and subepicardial (left-handed) myofibers results in a wringing motion or ‘twist’ of the LV around its long-axis during systole (24,25). When viewed from the apex, the LV performs a clockwise rotation at the base and a counterclockwise rotation at the apex (24,25).
LV twist has an important role in normal systolic and diastolic cardiac function (24,25). Reduced LV twist is commonly observed in patients with myocardial disease and LV dysfunction (27-31). LV apical or basal rotation may even be reversed with subsequent loss of LV twist, a so-called rigid body rotation (RBR) pattern (27). The importance of reversed apical rotation in adult patients with dilated cardiomyopathy (DCM) was demonstrated in a recent study by Popescu and co-workers (28). Adult patients with reversed apical rotation had more pronounced LV remodeling, ventricular dyssynchrony, and more severe cardiac dysfunction compared to DCM patients with reduced LV twist. A RBR pattern of LV rotation has been found not only in cardiomyopathies, but also in patients with CHD as well (29-31).
Abnormal myocardial fiber architecture in CHD
It should be noted that the basic myocardial fiber architecture in CHD seems different from that seen in normal hearts (13,32,33). These alterations have been observed in both morphologically right and left ventricles (32,33). The normal RV has only a small epicardial layer of circumferentially oriented fibers; the majority of the RV consists of deep longitudinal layers of myocardial fibers (13). In contrast, an additional middle layer of circular fibers has been shown in the myocardium of the RV in patients with TOF, suggesting that the alterations in the basic myocardial fiber architecture of the RV contributed to the differences in RV shape, frequently seen in these patients (32). Changes in myofiber and connective tissue architecture have also been demonstrated in patients with tricuspid atresia (33). Although the exact consequences of the alterations in myofiber architecture on deformation parameters are not known, it is speculated that this may contribute to the development of ventricular dysfunction in CHD.
3D-TTE: clinical applications
Real-time 3D-TTE provides more accurate volumetric data sets and better delineation of spatial relationships, which seems essential in understanding adult CHD and complementing 2D echocardiographic findings (12,34-36). Studies have shown that 3D-TTE measurements of ventricular volume and mass are comparable with those obtained by CMR (34-36). Furthermore, 3D-TTE has allowed a real-time visualization of the heart from a single volume acquisition, without the need for offline processing or reconstruction (34-36). The ease of data acquisition and decreased emphasis on expertise-driven interpretation has laid the foundation for the use of 3D echocardiography in clinical practice (34-36). However, 3D echocardiography is (still) limited by its less-than-optimal frame rates.
Clinical applications of strain and 3D echocardiography in congenital heart disease
TOF
TOF is the most common cyanotic heart defect, affecting nearly 10% of children with CHD (37). Advances in surgical strategies and perioperative management have dramatically improved outcome, with more than 90% of patients reaching adulthood (37,38). Nevertheless, long-term survival remains lower than in the general population. The difference in survival is commonly attributed to RV or LV dysfunction and sudden cardiac death caused by sustained ventricular tachyarrhythmias commonly arising from the right ventricular outflow tract (38). The overall incidence of sudden death has been estimated to be 1.2% at 10 years of follow-up (39). Although the arrhythmogenic burden is substantial, heart failure and exercise intolerance also have an important impact on morbidity and mortality (40-42). Therefore, identifying adult TOF patients at highest risk for major adverse events is a high priority research topic (40,41,43). Deformation imaging may provide additional information on myocardial function, which can be used for risk stratifying purposes in TOF (44,45).
Global and segmental RV dysfunction in TOF
RV dysfunction is significantly associated with adverse events and clinical outcome (40-45). Consequently, most effort has been put on unraveling the mechanisms contributing to RV remodeling and dysfunction in TOF (40-45). Impaired RV longitudinal strain (Figure 1) has been suggested as an early surrogate marker of RV dysfunction (4). Moreover, severity of pulmonary regurgitation, which is currently considered a major cause of RV dysfunction, may negatively impact RV strain measurements (4,46). Hence, an increasing number of studies used STE to assess RV function, demonstrating that global and regional longitudinal RV strain is decreased in both children and adults with repaired TOF (4,31,46-49). The impact of pulmonary valve replacement on RV longitudinal strain in repaired TOF has been studied as well (4). Although initial studies observed an increase in RV longitudinal strain after pulmonary valve replacement, no change in RV longitudinal strain was found in other studies (4,50-52).
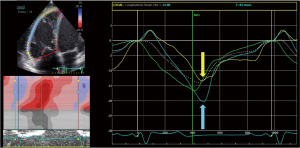
A specific strain pattern of reduced RV longitudinal strain has been identified, showing a progressive decrease in RV longitudinal strain from the base to the apex of the RV, with apical myocardial segments being most affected (4,46,47).
Normally, there is a progressive increase in RV longitudinal strain from the base to the apex of the RV (46,47). The clinical implication of this latter finding is somewhat unclear. A similar strain pattern has been recognized in other cardiovascular disease with compromised RV function as well, suggesting that impaired apical RV longitudinal strain might precede more global RV dysfunction (53,54). Thus, segmental apical RV function may be a promising marker for early RV dysfunction (46).
Although strain and strain rate measures are relatively load-independent in comparison to more conventional parameters of myocardial function, RV geometry and loading should be taken into account when interpreting RV strain parameters in repaired TOF (26,46). Indeed, a strong correlation between RV longitudinal strain and RV length was recently demonstrated in TOF patients (46).
LV function in TOF
A landmark study on the predictive value of LV systolic function in TOF patients revealed that moderate to severe LV systolic dysfunction is an important predictor of sudden cardiac death in adult patients late after TOF repair (55). Although the etiology of LV systolic dysfunction remains unclear, and is likely multifactorial, the authors identified older age at repair as a risk factor for LV systolic dysfunction and speculated that longer periods of volume overload and chronic hypoxemia, may, in part, explain the degree in LV dysfunction found in these patients (55). Moreover, they also hypothesized that, while adverse RV remodeling and dysfunction provide the substrate for ventricular arrhythmias, it is the severity of LV systolic dysfunction that ultimately may dictate the prognosis of ventricular arrhythmias in TOF patients. Other risk factors associated with poor LV systolic function were shunt duration, presence of RV dysfunction and recurrent arrhythmias (56). In addition, LV diastolic dysfunction also seems a risk factor for worse clinical outcome (57). Taken together, LV systolic and diastolic function may represent promising additional parameters to predict the development of adverse events in this challenging patient group (55,57).
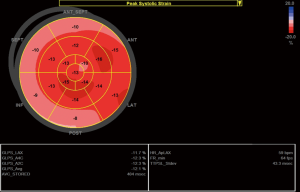
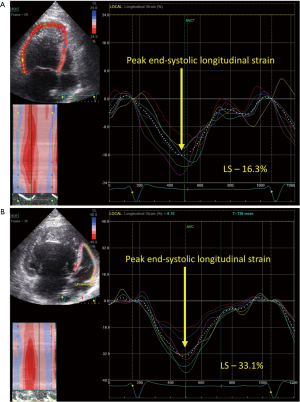
However, although LV dysfunction is relatively common in TOF patients, only few patients will present with more than mildly reduced LV systolic function (56,58). Therefore, more sensitive echocardiographic measures are needed to identify early LV myocardial dysfunction, before a significant decline in LV ejection fraction is noted in these patients (Figure 2). Diller et al. used global LV longitudinal strain (GLS) as an early and sensitive measure of LV systolic dysfunction in a multicenter study on TOF outcome (58). The authors demonstrated that systolic LV dysfunction, measured as lower GLS values, in addition to other echocardiographic variables, was associated with a greater risk of major adverse events (58). Global LV longitudinal strain is a novel and powerful predictive strain parameter and seems feasible for use in daily practice.
Electromechanical delay in TOF
Electromechanical dyssynchrony of the RV (and LV) is common after repair of TOF and is associated with the postoperative right bundle branch block (RBBB) (43,59-63). Ventricular dyssynchrony increases over time in most patients. Although ventricular dyssynchrony has been largely ignored as an important contributor to clinical outcome of TOF patients in earlier studies, it is nowadays a well-recognized contributor to the pathophysiology of RV dysfunction (59-63). Moreover, cardiac resynchronization therapy (CRT) has been applied to right-sided disease in CHD (60,64,65). Before discussing this exciting topic, some background information on ventricular dyssynchrony is required to fully appreciate the importance of electromechanical delay in TOF patients, and patients with other types of CHD.
The findings of the multicenter PROSPECT trial from 2008 resulted in a fierce debate on the role of echocardiography in the assessment of ventricular dyssynchrony in patients with heart failure and reduced LV ejection fraction (66). This landmark study failed to demonstrate a benefit of echocardiographic assessment of mechanical dyssynchrony in predicting clinical response after CRT. Particularly the reproducibility of tissue Doppler imaging-derived dyssynchrony indices was of concern in this prospective study (66). It should be noted that there is an important distinction between mechanical dyssynchrony and electrical dyssynchrony (67-70). New insights in the pathophysiologic mechanics of ventricular dyssynchrony showed that the ‘classical’ assessment of LV mechanical delay between ventricular walls by echocardiography is not only dependent on LV electrical depolarization delay (electrical dyssynchrony), but also on abnormalities in regional contractility and loading condition of the failing ventricle (67-70). Recent work from the group of Lumens et al., who used a well-established computer-based model (Circ-Adapt), clearly showed the conceptual distinction between mechanical discoordination and electrical dyssynchrony in patients with heart failure (68). Only patients with patterns of LV mechanical discoordination caused by electromechanical substrates, and not regional differences in contractility or scar tissue, responded favorably to CRT (68). Additional reports have been published, including electroanatomical mapping studies, to further define and broaden these electromechanical concepts not only in patients with heart failure, but also in patients with CHD (67-71).
The concepts mentioned earlier highlighted the need for the development of novel electromechanical dyssynchrony indices, responsive to CRT. In recent years, cardiac imaging has been very helpful to identify dyssynchrony patterns that are related to an electromechanical delay (67). Most of these indices are based on speckle tracking techniques and all have been validated in large clinical cohort studies (67).
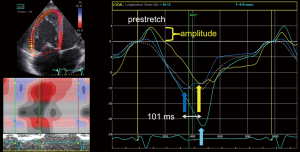
A recent study in TOF demonstrated that RV prestretch amplitude and duration, postsystolic strain, and RV lateral-septal delay quantify the severity of RV electromechanical dyssynchrony (Figure 3) (61). The RV basal wall was the latest and the mid and apical interventricular septal segments the earliest contracting myocardial segments, reflecting the underlying electromechanical pathophysiology. Because it is not feasible to use the response to CRT as a clinical outcome measure, the authors studied the relationship between these novel dyssynchrony indices and clinical parameters such as RV function, arrhythmias and exercise capacity (61). It was shown that all dyssynchrony markers correlated to some degree with RV EF and RV global longitudinal strain. Moreover, the prestretch amplitude and duration were found to be simple measures of RV dyssynchrony in TOF patients, with good reliability and reproducibility (61). Taken together, studying electromechanical delay of the RV in repaired TOF patients seems important to understand the pathophysiological mechanisms driving RV dysfunction. This information may be used to select those TOF patients with RV failure who may potentially benefit from CRT. However, larger studies are needed to confirm these preliminary findings (60,64,72).
Rotational mechanics of the LV in TOF
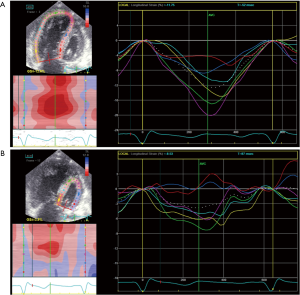
LV rotational abnormalities are common after TOF repair (31,48,50,73,74). Reduced twist of the LV has repeatedly been observed in TOF patients following repair (Figure 4) (31,48,50,73,74). Of importance, several groups identified an RBR pattern consisting of reversed basal clockwise (= counterclockwise) rotation, with subsequent loss of normal LV twist, in up to 38% of patients with TOF (31,50,73). This RBR pattern of reversed basal rotation was associated with lower global RV longitudinal strain and GLS (31,50). On multivariate regression analysis, the only significant predictor of counterclockwise basal rotation was RV longitudinal strain (31). The authors suggested that RV function, more than RV dilatation influences abnormal LV twist. However, the mechanisms involved may be multifactorial and are largely unclear (31,50,73). Furthermore, the same group studied the LV mechanics of a cohort of TOF patients undergoing pulmonary valve replacement (50). Those with a pre-operative reversed basal rotation pattern had significantly lower global RV longitudinal strain values early post-operative, and decreased GLS at mid-term follow-up. In 42% of patients with reversed basal rotation, this RBR pattern reverted to normal clockwise basal rotation during follow-up. Nonetheless, there were no significant differences in conventional echocardiographic and longitudinal strain parameters of the RV and LV between patients in whom normalization of basal rotation was noted compared to those who had persistently abnormal basal rotation (50). Thus, the impact of LV rotational mechanics on RV function and remodeling highlights the presence of ventriculo-ventricular interaction. Changes in the geometry of the RV, due to remodeling and volume overload, may induce alterations in the delicate myofiber architecture of the interventricular septum and other parts of the heart, that could lead to a distortion of the LV performance and vice versa (48). More recently, Yim et al. demonstrated a strong correlation between the severity of RV and LV diffuse myocardial fibrosis in TOF patients on CMR imaging, using native T1 mapping (75). These findings support the concept that ventriculo-ventricular interactions also occur at the (myocardial) tissue level.
Real-time 3D echocardiography of the RV in TOF
Assessing RV remodeling and function using 3D echocardiography in TOF patients has been a specific area of interest (4,12). The feasibility of 3D echocardiography in TOF is largely determined by having a good echocardiographic window, which can be challenging in patients with severe dilation of the RV. In essence, it is very important to include the whole RV in a single acquisition, otherwise 3D echo evaluation results in an underestimation of RV volumes, thereby limiting its clinical applicability (4,12). New developments in this field may overcome these limitations.
The systemic RV after atrial switch repair and double discordance
Simple transposition of the great arteries (TGA, or D-TGA) palliated with an atrial switch type operation (Mustard or Senning) and congenitally corrected TGA (double discordance, Figure 5) are the most common examples of CHD with a biventricular physiology in which the RV supports the systemic circulation (76,77). These patients are commonly encountered in adult CHD clinics, and management of these patients remains challenging due to development of progressive systemic RV dysfunction, heart failure, life-threatening arrhythmias, and sudden cardiac death (76,77). Systemic RV failure is associated with increased morbidity and poor prognosis (76,77). Several palliative therapies, such as pulmonary artery banding, CRT implantation, or tricuspid valve replacement in those with RV failure and severe regurgitation, have been adopted to improve functional status (76-78). However, heart transplantation currently remains the only long-term lifesaving treatment in adults with end-stage systemic RV failure (76,77).
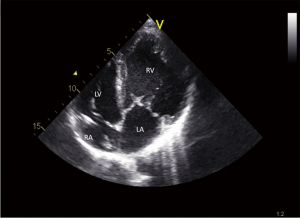
Subaortic and subpulmonic global ventricular function
Clinical overt systemic RV dysfunction is frequently encountered in adult patients, indicating that the RV is unable to support the systemic circulation during long-term follow-up. In most patients’ subclinical RV systolic dysfunction is already present at a much younger age, underscoring the need for close echocardiographic follow-up (77).
STE has been used in a relatively small number of studies to investigate RV and LV myocardial deformation and mechanics (Figure 6) (79-82). These studies have confirmed that global RV peak systolic longitudinal strain is significantly reduced in patients with a systemic RV (atrial switch, ccTGA) (79-82). Moreover, there seems a shift from a predominantly longitudinal to a more circumferential myocardial contraction pattern in the systemic RV of these patients (83,84). Because myofibers are predominantly longitudinally arranged in the normal RV, and long-axis shortening is the main contributor to RV performance, this finding suggests that the change in contraction pattern seems an adaptive response to the increased afterload of a RV in a subaortic position (80,83,84). Furthermore, the subpulmonic ventricle also seems affected; global LV longitudinal strain is impaired in patients with TGA after atrial switch operation, whereas, preserved GLS was found in most patients with ccTGA (80).
Ventriculo-ventricular interaction in TGA
Strain assessment of the systemic RV and the subpulmonic LV suggests that these functions are interrelated (80,85). This correlation was confirmed by measuring RV and LV ejection fractions on CMR (80), and is a well-recognized hemodynamic feature in patients after repaired TOF. This ventriculo-ventricular interaction is more pronounced in patients with ccTGA compared to those after atrial switch. Notably, the relationship between RV and LV global longitudinal strain is much stronger in ccTGA with a significant pulmonary stenosis than in ccTGA patients without a relevant pulmonary stenosis (80). The physiological mechanism behind this observation is probably that a higher LV afterload (pulmonary stenosis) decreases the leftward shift of the interventricular septum, thereby improving RV geometry and function (80). A similar phenomenon has been observed in TGA patients undergoing pulmonary artery banding in preparation for an arterial switch operation who developed systemic RV failure following their arterial switch, in TOF patients with a residual outflow tract obstruction or pulmonary stenosis after repair, and more recently, in patients with dilated cardiomyopathy and end-stage heart failure undergoing pulmonary arterial banding to improve LV function (80,86,87).
Diastolic systemic RV function
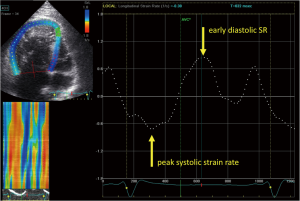
Diastolic function of the systemic RV has also been studied by measuring global RV early diastolic strain rate (Figure 7) (79,85). Although impaired diastolic function of the systemic RV is present in most TGA patients after atrial switch, the clinical importance of diastolic function remains unclear at this point (79,85). Part of this uncertainty is probably the result of the poor reproducibility of early diastolic strain rate compared to global RV strain in patients with TGA after atrial switch, despite the theoretical advantage of strain rate being more load independent (79).
Clinical applications of strain imaging in systemic RV disease
In addition to these important findings, there have been several potential clinical implications in the routine use of deformation imaging in these patients. In a recent CMR study of patients with a systemic RV, global RV longitudinal strain was the best echocardiographic parameter of identifying patients with a RV EF of <45%, with a sensitivity and specificity of 77.3% and 72.7%, respectively, when using a cutoff strain value of <−16.3% (88). Other investigators have shown that global RV longitudinal strain correlated moderately well with exercise capacity, which is an important predictor of clinical outcome in patients with CHD (89). Interestingly, global RV longitudinal strain has been identified as a prognostic marker of adverse events during follow-up in patients with a systemic RV, independently of a history of arrhythmias and advanced NYHA functional class, which are important established clinical predictors of late-mortality (79,80).
CRT and electromechanical dyssynchrony in systemic RV
Systemic RVs demonstrate increased electromechanical dyssynchrony (90,91). The ventricular dyssynchrony is related to the ventricular conduction abnormalities frequently observed in these patients, such as intrinsic RBBB or pacing induced RBBB for complete heart block (90,91). Notably, RBBB often results in progressive RV dyssynchrony and subsequent RV dysfunction (76-78). Moreover, there is a strong inverse correlation between the severity of electromechanical delay and the degree of RV dysfunction in patients with a systemic RV (90,91). Consequently, studies have shown that a subgroup of patients with a systemic RV and biventricular physiology may benefit from CRT. However, there is a high non-response rate of patients undergoing CRT, highlighting the need for specific predictive markers for CRT response in systemic RVs (90,91). This major clinical problem has also been recognized in patients with other types of CHD (76-78,90,91).
A ‘classic’ pattern of electromechanical delay has been identified using strain imaging, which has been covered in part in the previous section on TOF. The presence of this typical strain pattern seems predictive of both short- and long-term CRT response in various patient populations (67,91). The echocardiographic characteristics of this electromechanical pattern consists of early septal activation, opposed by early stretch in the activation-delayed RV free wall, followed by a late RV free wall contraction with early termination of septal contraction (59,67,91). Although electrical abnormalities can be observed in most TGA patients with a systemic RV, the classic-pattern of electromechanical delay was present in nearly half of the patients investigated with STE in a recent study (91). Of importance, this typical strain pattern was highly reproducible between readers, was associated with moderate to severe RV systolic dysfunction, and indicates a potential benefit from CRT in these patients (91). However, this approach should be tested in a prospective study.
3D echocardiography in simple TGA following atrial switch and ccTGA
3D echocardiography has been applied to comprehensively analyze the shape, volume, global and regional function of the systemic RV in these patients. Not surprisingly, the RV is more dilated, rounded, and impaired in function compared to healthy controls (92,93). Kutty et al. used a new imaging tool based on the piecewise smooth subdivision surface technology to quantify the systemic RV in TGA patients (94). This technique creates a ‘knowledge-based’ reconstruction of the RV after identifying key anatomic landmarks (93,94). A 3D surface is then created using a big database that contains knowledge of the shapes of normal and abnormal human RVs (93,94). An advantage of knowledge-based reconstruction is that identification of entire endocardial borders is not necessary for reconstruction of the RV. Excellent feasibility and reproducibility was recently demonstrated for the assessment of systemic RV size and function in adult patients with TGA (94). This technique holds promise for serial follow-up assessments of RV size and function (93,94).
Fontan palliation of the single ventricle
The total cavo-pulmonary connection (TCPC) is a staged surgical palliation used to treat patients with single ventricle physiology (95-97). Creating the TCPC using an extracardiac conduit is currently the most preferred surgical strategy to establish the Fontan circulation (95-97). This final surgical stage is often accomplished several years after a bidirectional Glenn connection, in which the bloodflow from the superior vena cava is re-routed to the pulmonary arteries (6,95). After this procedure, the systemic venous return flows directly to the pulmonary arteries (e.g., Fontan circulation) (6,96).
Despite improved surgical techniques and patient care, Fontan patients continue to have high long-term morbidity and mortality (96-100). It has become clear that the Fontan circulation affects most of the organs in the human body, with low cardiac output and chronically elevated systemic venous pressure likely being the substrates for the development of adverse effects (98-101). Significant complications include the development of progressive ventricular dysfunction, exercise intolerance, liver fibrosis, arrhythmias, protein-losing enteropathy, and plastic bronchitis (97-101). Therefore, close monitoring of these patients and serial assessment of the morphology and functional capacity of the single ventricle is warranted (5,6). Moreover, TTE, particularly when emphasizing the potential of deformation imaging and 3D-echocardiography, plays a major role in the identification of patients at highest risk for major adverse events (5,6).
Ventricular dysfunction in Fontan patients
Progressive ventricular dysfunction is relatively common in adult Fontan patients, and is associated with poor patient outcome (102-104). Because a proper ventricular pump on the pulmonary site of the circulation is missing, the Fontan circulation is particularly vulnerable to low cardiac output when the systemic ventricle fails (5,6). Although recent data from several long-term outcome studies suggest that ventricular systolic function seems preserved in the majority of Fontan patients with single left or right ventricles, most Fontan patients will have echocardiographic evidence of diastolic dysfunction (5,6,102-104). It should be noted that even mild diastolic dysfunction may cause a significant elevation of the ventricular filling pressures, limiting the capacity of the single ventricle to maintain a sufficient cardiac output. Indeed, several studies have demonstrated a contractility-afterload mismatch in Fontan patients, suggesting that diastolic function may play a key role in the development of a failing Fontan circulation (105-107). Furthermore, the etiology of ventricular dysfunction in Fontan patients is not well understood and is probably multifactorial (5,6,13,33). Abnormal myofiber architecture, myocardial fibrosis, pre-Fontan cyanosis, volume overload, endocardial fibroelastosis, electromechanical dyssynchrony, multiple surgical procedures, activation of the renin-angiotensin-aldosterone system, systemic inflammation, and end-organ damage may all contribute to the development of (subclinical) ventricular dysfunction (5,6,13,33,100,108).
Deformation imaging in Fontan
The assessment of the systolic function of single ventricles is challenging due to the heterogeneity in intracardiac morphology and loading conditions (5,6). Notably, the evaluation of RV function is particularly difficult due to the non-geometric shape of this ventricle. Consequently, ventricular function is routinely assessed using a subjective evaluation (e.g., ‘eyeballing’) or application of the biplane Simpson’s methodology and fractional area of change (FAC) technique in case of a morphologic left or right/indeterminate single ventricle, respectively (5,6,109). Therefore, deformation imaging may aid in the identification and quantification of myocardial dysfunction and electromechanical delay, which may have therapeutic implications for the management of progressive ventricular systolic dysfunction and heart failure in this group of patients (5,6,109).
Circumferential and longitudinal strain is reduced in most Fontan patients with preserved LV EF, indicating the presence of subclinical or early myocardial dysfunction (110,111). In addition, global strain of the systemic RV in single ventricle physiology is also impaired (Figure 8) (107,112). Strain-derived parameters are highly reproducible and have been validated against CMR obtained measures of cardiac function in several different cohorts of patients with single ventricle physiology (111,113). However, global strain parameters, such as GLS and GRS, are more robust measures of ventricular performance than regional strain parameters. Moreover, the reliability of strain measurements seems affected by frame rate, the nature of strain (longitudinal, circumferential, radial), and even ventricular geometry, suggesting that a dedicated echocardiographic protocol is recommended for strain analysis of the single ventricle in a clinical setting (111).
Another promising clinical application of deformation imaging is the use of strain measures to predict the postoperative course of patients undergoing a TCPC operation (114). Park et al. showed in a cohort of 135 single ventricle patients that preoperative circumferential strain rate was independently associated with length of hospital stay (LOS) >14 days (114). Patients with a preoperative circumferential strain rate of < −1.5 s−1 were at very low risk for a prolonged LOS, and patients with a circumferential strain rate of > −1.0 s−1 were considered high-risk candidates (114). Whether optimization of preoperative heart failure treatment may contribute to better postoperative outcomes remains to be investigated. Current findings suggest that deformation imaging has the potential to allow the clinician to reliably assess the contribution of myocardial function to short-term and long-term outcome in Fontan survivors.
Ventricular dyssynchrony in single ventricles
Significant electromechanical dyssynchrony with early activation of a myocardial segment or segments and subsequent pre-stretch of the opposing myocardial segment has been identified in Fontan patients, particularly in those with permanent pacing therapy and intraventricular conduction delays (115,116). In this regard, the hypoplastic left heart syndrome (HLHS) population is relatively well studied using various echocardiographic modalities, including STE (71,115-117). Several studies have demonstrated the presence of mechanical dyssynchrony in the majority patients with HLHS (71,115-117). This finding has led to increased interest in CRT to improve RV function in these patients (71,118,119). Conflicting results of CRT-response have been reported, suggesting that only a certain subgroup of HLHS patients have an electrical substrate amenable to CRT. A recent study by Motonaga et al. confirmed the presence of mechanical dyssynchrony in HLHS patients with preserved systolic RV function using invasive electroanatomic mapping (71). Nevertheless, the authors found no intrinsic substrate for electrical dyssynchrony in these patients. Interestingly, classic-pattern dyssynchrony, which is a strong predictor of CRT-response, could be demonstrated by STE in a small proportion of investigated Fontan patients (116). The presence of classic-pattern dyssynchrony was associated with impaired systolic and diastolic function, including reduced circumferential strain, longitudinal strain and early diastolic strain rate, compared with Fontan patients without this pattern of electromechanical delay (116). The QRS duration was also increased in these patients. Because the presence of classic-pattern dyssynchrony seems a very promising marker for CRT-response, further work is needed to confirm these preliminary findings (116).
3D-echocardiography in single ventricle physiology
Only a small number of studies have used real-time 3D echocardiography to evaluate systemic function and anatomic considerations of atrioventricular valves in patients with single ventricle physiology. Most of the studied patients were infants and children (120-122). Mechanisms of tricuspid valve regurgitation in children with HLHS have extensively been studied using 3D echocardiography (121,122). However, 3D echocardiography is not routinely being used in adult Fontan patients.
Future directions
Machine learning techniques
Recently, machine learning techniques have been introduced to the field of cardiac imaging (123-125). Although echocardiography is widely used in daily practice, it is highly operator-dependent and requires extensive training for correct interpretation of the acquired data (124). A key feature of machine learning is that the algorithm recognizes patterns within large data sets and is able to learn from input of additional variables in time to make better predictions on, for example, patient outcome and development of adverse events (123-125). Samad et al. applied machine learning to a baseline data set of clinical and CMR variables obtained from adult patients with repaired TOF (123). They were able to identify adult TOF patients at risk for clinical deterioration during follow-up. Thus, incorporating machine learning methodologies in cardiac imaging may have important implications for clinical-decision making and management of patients with CHD.
Ultrafast cardiac ultrasound imaging
Technical advances in ultrasound techniques allow images to be acquired at a very high frame rate (126,127). This so-called ultrafast, or high frame-rate imaging, has the potential to provide more insight into myocardium contractility, particularly during the very short-lived isovolumetric and relaxation periods, and cardiac blood flow properties (126). Furthermore, myocardial stiffness can be evaluated by assessing the propagation characteristics of shear waves through the myocardial tissue (126). Notably, STE has successfully been applied to images obtained with high frame-rate echocardiography (127). To the best of our knowledge, there are no studies using these novel techniques in the field of adult CHD yet.
Conclusions
Although several issues need further investigation before strain imaging and 3D echocardiography can become mainstream modalities for the quantitative assessment of ventricular function and morphology, respectively, both techniques seem very promising additional tools in our extending armamentarium for adult CHD. Moreover, strain imaging provides a more sensitive method for detecting subclinical alterations in ventricular systolic and diastolic function that may otherwise be missed with conventional echocardiographic measurements. Global longitudinal strain is the deformation parameter of choice, because it is reproducible, feasible to obtain in a clinical setting, and has a good predictive value in CHD. Real-time 3D echocardiography seems very useful to assess ventricular volumes, ejection fraction, valvular morphology and intracardiac anatomy. Development and validation of dedicated echocardiographic protocols for each specific CHD lesion incorporating the use of deformation imaging and 3D-echocardiography is strongly recommended.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Burchill LJ, Huang J, Tretter JT, et al. Noninvasive imaging in adult congenital heart disease. Circ Res 2017;120:995-1014. [Crossref] [PubMed]
- Gaydos SS, Varga-Szemes A, Judd RN, et al. Imaging in adult congenital heart disease. J Thorac Imaging 2017;32:205-16. [Crossref] [PubMed]
- Babu-Narayan SV, Giannakoulas G, Valente AM, et al. Imaging of congenital heart disease in adults. Eur Heart J 2016;37:1182-95. [Crossref] [PubMed]
- Larios G, Friedberg MK. Imaging in repaired tetralogy of Fallot with a focus on recent advances in echocardiography. Curr Opin Cardiol 2017;32:490-502. [Crossref] [PubMed]
- Grattan M, Mertens L. Mechanics of the functionally univentricular heart – how little do we understand and why does it matter? Can J Cardiol 2016;32:1033.e11-8. [Crossref] [PubMed]
- Kutty S, Rathod RH, Danford DA, et al. Role of imaging in the evaluation of single ventricle with the Fontan palliation. Heart 2016;102:174-83. [Crossref] [PubMed]
- Tworetzky W, McElhinney DB, Brook MM, et al. Echocardiographic diagnosis alone for complete repair of major congenital heart defects. J Am Coll Cardiol 1999;33:228-33. [Crossref] [PubMed]
- Benavidez OJ, Gauvreau K, Jenkins KJ, et al. Diagnostic errors in pediatric echocardiography: development of taxonomy and identification of risk factors. Circulation 2008;117:2995-3001. [Crossref] [PubMed]
- Valente AM, Cook S, Festa P, et al. Multimodality imaging guidelines for patients with repaired tetralogy of Fallot: a report from the American Society of Echocardiography: developed in collaboration with the Society for Cardiovascular Magnetic Resonance and the Society for Pediatric Radiology. J Am Soc Echocardiogr 2014;27:111-41. [Crossref] [PubMed]
- Cohen MS, Eidem BW, Cetta F, et al. Multimodality imaging guidelines of patients with transposition of the great arteries: a report from the American Society of Echocardiography in collaboration with the Society for Cardiovascular Magnetic Resonance and the Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 2016;29:571-621. [Crossref] [PubMed]
- Lopez L, Colan SD, Frommelt PC, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography and Congenital Heart Disease Council. J Am Soc Echocardiogr 2010;23:465-95. [Crossref] [PubMed]
- Simpson J, Lopez L, Acar P, et al. Three-dimensional echocardiography in congenital heart disease: an expert consensus document from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr 2017;30:1-27. [Crossref] [PubMed]
- Stout KK, Broberg CS, Book WM, et al. Chronic heart failure in congenital heart disease: a scientific statement from the American Heart Association. Circulation 2016;133:770-801. [Crossref] [PubMed]
- Oechslin EN, Harrison DA, Connelly MS, et al. Mode of death in adults with congenital heart disease. Am J Cardiol 2000;86:1111-6. [Crossref] [PubMed]
- Verheugt CL, Uiterwaal CS, van der Velde ET, et al. Mortality in adult congenital heart disease. Eur Heart J 2010;31:1220-9. [Crossref] [PubMed]
- Margossian R, Schwartz ML, Prakash A, et al. Comparison of echocardiographic and cardiac magnetic resonance imaging measurements of functional single ventricular volumes, mass, and ejection fraction. Am J Cardiol 2009;104:419-28. [Crossref] [PubMed]
- Colquitt JL, Pignatelli RH. Strain imaging: the emergence of speckle tracking echocardiography into clinical pediatric cardiology. Congenit Heart Dis 2016;11:199-207. [Crossref] [PubMed]
- Collier P, Phelan D, Klein A. A test in context: myocardial strain measured by speckle-tracking echocardiography. J Am Coll Cardiol 2017;69:1043-56. [Crossref] [PubMed]
- Forsey J, Friedberg MK, Mertens L. Speckle tracking echocardiography in pediatric and congenital heart disease. Echocardiography 2013;30:447-59. [Crossref] [PubMed]
- Muraru D, Niero A, Rodriguez-Zanella H, et al. Three-dimensional speckle tracking echocardiography: benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc Diagn Ther 2018;8:101-17. [Crossref] [PubMed]
- Geyer H, Caracciolo G, Abe H, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr 2010;23:351-69. [Crossref] [PubMed]
- Blessberger H, Binder T. Non-invasive imaging: two-dimensional speckle tracking echocardiography: basic principles. Heart 2010;96:716-22. [Crossref] [PubMed]
- Pavlopoulos H, Nihoyannopoulos P. Strain and strain rate deformation patterns: from tissue Doppler to 2D speckle tracking. Int J Cardiovasc Imaging 2008;24:479-91. [Crossref] [PubMed]
- Sengupta PP, Korinek J, Belohlavek M, et al. Left ventricular structure and function: basic science for cardiac imaging. J Am Coll Cardiol 2006;48:1988-2001. [Crossref] [PubMed]
- Sengupta PP, Tajik AJ, Chandrasekaran K, et al. Twist mechanics of the left ventricle: principles and application. JACC Cardiovasc Imaging 2008;1:366-76. [Crossref] [PubMed]
- Bijnens B, Cikes M, Butakoff C, et al. Myocardial motion and deformation: what does it tell us and how does it relate to function? Fetal Diagn Ther 2012;32:5-16. [Crossref] [PubMed]
- van Dalen BM, Caliskan K, Soliman OI, et al. Left ventricular solid body rotation in non-compaction cardiomyopathy: a potential new objective and quantitative functional diagnostic criterion? Eur J Heart Fail 2008;10:1088-93. [Crossref] [PubMed]
- Popescu BA, Beladan CC, Calin A, et al. Left ventricular remodelling and torsional dynamics in dilated cardiomyopathy: reversed apical rotation as a marker of disease severity. Eur J Heart Fail 2009;11:945-51. [Crossref] [PubMed]
- Udink ten Cate FE, Schmidt BE, Lagies R, et al. Reversed apical rotation and paradoxical increased left ventricular torsion in children with left ventricular non-compaction. Int J Cardiol 2010;145:558-9. [Crossref] [PubMed]
- Udink ten Cate FE, Lorenz SRW, Khalil M, et al. Does reversed apical or basal left ventricular rotation in children with dilated cardiomyopathy recover following medical therapy? A two-dimensional speckle tracking study. Int J Cardiol 2011;153:330-3. [Crossref] [PubMed]
- Dragulescu A, Friedberg MK, Grosse-Wortmann L, et al. Effect of chronic right ventricular volume overload on ventricular interaction in patients after tetralogy of Fallot repair. J Am Soc Echocardiogr 2014;27:896-902. [Crossref] [PubMed]
- Sanchez-Quintana D, Anderson RH, Ho SY. Ventricular myoarchitecture in tetralogy of Fallot. Heart 1996;76:280-6. [Crossref] [PubMed]
- Sanchez-Quintana D, Climent V, Ho SY, et al. Myoarchitecture and connective tissue in hearts with tricuspid atresia. Heart 1999;81:182-91. [Crossref] [PubMed]
- Lang RM, Mor-Avi V, Sugeng L, et al. Three-dimensional echocardiography: the benefits of the additional dimension. J Am Coll Cardiol 2006;48:2053-69. [Crossref] [PubMed]
- Lang RM, Tsang W, Weinert L, et al. Valvular heart disease: the value of 3-dimensional echocardiography. J Am Coll Cardiol 2011;58:1933-44. [Crossref] [PubMed]
- Vettukattil JJ. Three dimensional echocardiography in congenital heart disease. Heart 2012;98:79-88. [Crossref] [PubMed]
- Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. Second of two parts. N Engl J Med 2000;342:334-42. [Crossref] [PubMed]
- Murphy JG, Gersh BJ, Mair DD, et al. Long-term outcome in patients undergoing surgical repair of tetralogy of Fallot. N Engl J Med 1993;329:593-9. [Crossref] [PubMed]
- Silka MJ, Hardy BG, Menashe VD, et al. A population-based prospective evaluation of risk of sudden cardiac death after operation for common congenital heart defects. J Am Coll Cardiol 1998;32:245-51. [Crossref] [PubMed]
- Khairy P, Aboulhosn J, Gurvitz MZ, et al. Arrhythmia burden in adults with surgically repaired tetralogy of Fallot: a multi-institutional study. Circulation 2010;122:868-75. [Crossref] [PubMed]
- Geva T. Repaired tetralogy of Fallot: the roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. J Cardiovasc Magn Reson 2011;13:9. [Crossref] [PubMed]
- Villafañe J, Feinstein JA, Jenkins KJ, et al. Hot topics in tetralogy of Fallot. J Am Coll Cardiol 2013;62:2155-66. [Crossref] [PubMed]
- Udink ten Cate FE, Sreeram N, Brockmeier K. The pathophysiologic aspects and clinical implications of electrocardiographic parameters of ventricular conduction delay in repaired tetralogy of Fallot. J Electrocardiol 2014;47:618-24. [Crossref] [PubMed]
- DiLorenzo MP, Elci OU, Wang Y, et al. Longitudinal changes in right ventricular function in tetralogy of Fallot in the initial years after surgical repair. J Am Soc Echocardiogr 2018;31:816-21. [Crossref] [PubMed]
- Orwat S, Diller GP, Kempny A, et al. Myocardial deformation parameters predict outcome in patients with repaired tetralogy of Fallot. Heart 2016;102:209-15. [Crossref] [PubMed]
- Dragulescu A, Grosse-Wortmann L, Redington A, et al. Differential effect of right ventricular dilatation on myocardial deformation in patients with atrial septal defects and patients after tetralogy of Fallot. Int J Cardiol 2013;168:803-10. [Crossref] [PubMed]
- Menting ME, van den Bosch AE, McGhie JS, et al. Assessment of ventricular function in adults with repaired tetralogy of Fallot using myocardial deformation imaging. Eur Heart J Cardiovasc Imaging 2015;16:1347-57. [PubMed]
- van der Hulst AE, Delgado V, Holman ER, et al. Relation of left ventricular twist and global strain with right ventricular dysfunction in patients after operative ‘correction’ of tetralogy of Fallot. Am J Cardiol 2010;106:723-9. [Crossref] [PubMed]
- Scherptong RWC, Mollema SA, Blom NA, et al. Right ventricular peak systolic longitudinal strain is a sensitive marker for right ventricular deterioration in adult patients with tetralogy of Fallot. Int J Cardiovasc Imaging 2009;25:669-76. [Crossref] [PubMed]
- Yim D, Mertens L, Morgan CT, et al. Impact of surgical pulmonary valve replacement on ventricular mechanics in children with repaired tetralogy of Fallot. Int J Cardiovasc Imaging 2017;33:711-20. [Crossref] [PubMed]
- Gursu HA, Varan B, Sade E, et al. Analysis of right ventricle function with strain imaging before and after pulmonary valve replacement Cardiol J 2016;23:195-201. [Crossref] [PubMed]
- Balasubramanian S, Harrild DM, Kerur B, et al. Impact of surgical pulmonary valve replacement on ventricular strain and synchrony in patients with repaired tetralogy of Fallot: a cardiovascular magnetic resonance feature tracking study. J Cardiovasc Magn Reson 2018;20:37. [Crossref] [PubMed]
- Dambrauskaite V, Delcroix M, Claus P, et al. Regional right ventricular dysfunction in chronic pulmonary hypertension. J Am Soc Echocardiogr 2007;20:1172-80. [Crossref] [PubMed]
- Fernandez-Friera L, Garcia-Alvarez A, Guzman G, et al. Apical right ventricular dysfunction in patients with pulmonary hypertension demonstrated with magnetic resonance. Heart 2011;97:1250-6. [Crossref] [PubMed]
- Ghai A, Silversides C, Harris L, et al. Left ventricular dysfunction is a risk factor for sudden cardiac death in adults late after repair of tetralogy of Fallot. J Am Coll Cardiol 2002;40:1675-80. [Crossref] [PubMed]
- Broberg CS, Aboulhosn J, Mongeon FP, et al. Prevalence of left ventricular systolic dysfunction in adults with repaired tetralogy of Fallot. Am J Cardiol. 2011;107:1215-20. [Crossref] [PubMed]
- Khairy P, Harris L, Landzberg MJ, et al. Implantable cardioverter-defibrillators in tetralogy of Fallot. Circulation 2008;117:363-70. [Crossref] [PubMed]
- Diller GP, Kempny A, Liodakis E, et al. Left ventricular longitudinal function predicts life-threatening ventricular arrhythmia and death in adults with repaired tetralogy of Fallot. Circulation 2012;125:2440-6. [Crossref] [PubMed]
- Hui W, Slorach C, Dragulescu A, et al. Mechanisms of right ventricular electromechanical dyssynchrony and mechanical inefficiency in children after repair of tetralogy of Fallot. Circ Cardiovasc Imaging 2014;7:610-8. [Crossref] [PubMed]
- Janoušek J, Kovanda J, Ložek M, et al. Pulmonary right ventricular resynchronization in congenital heart disease: acute improvement in right ventricular mechanics and contraction efficiency. Circ Cardiovasc Imaging 2017.10. [PubMed]
- Yim D, Hui W, Larois G, et al. Quantification of right ventricular electromechanical dyssynchrony in relation to right ventricular function and clinical outcomes in children with repaired tetralogy of Fallot. J Am Soc Echocardiogr 2018;31:822-30. [Crossref] [PubMed]
- Abd El Rahman MY, Hui W, Yigitbasi M, et al. Detection of left ventricular asynchrony in patients with right bundle branch block after repair of tetralogy of Fallot using tissue-Doppler imaging-derived strain. J Am Coll Cardiol 2005;45:915-21. [Crossref] [PubMed]
- Bordachar P, Iriart X, Chabaneix J, et al. Presence of ventricular dyssynchrony and haemodynamic impact of right ventricular pacing in adults with repaired tetralogy of Fallot and right bundle branch Block. Europace 2008;10:967-71. [Crossref] [PubMed]
- Thambo JB, Dos Santos P, De Guillebon M, et al. Biventricular stimulation improves right and left ventricular function after tetralogy of Fallot repair: acute animal and clinical studies. Heart Rhythm 2010;7:344-50. [Crossref] [PubMed]
- Karpawich PP, Bansal N, Samuel S, et al. 16 years of cardiac resynchronization pacing among congenital heart disease patients: direct contractility (dP/dt-max) screening when the guidelines do not apply. JACC Clin Electrophysiol 2017;3:830-41. [Crossref] [PubMed]
- Chung ES, Leon AR, Tavazzi L, et al. Results of the predictors of response to CRT (PROSPECT) trial. Circulation 2008;117:2608-16. [Crossref] [PubMed]
- Marechaux S, Menet A, Guyomar Y, et al. Role of echocardiography before cardiac resynchronization therapy: new advances and current developments. Echocardiography 2016;33:1745-52. [Crossref] [PubMed]
- Lumens J, Tayal B, Walmsley J, et al. Differentiating electromechanical from non-electrical substrates of mechanical discoordination to identify responders to cardiac resynchronization therapy. Circ Cardiovasc Imaging 2015;8:e003744. [Crossref] [PubMed]
- Auricchio A, Lumens J, Prinzen FW. Does cardiac resynchronization therapy benefit patients with right bundle branch block: cardiac resynchronization therapy has a role in patients with right bundle branch block. Circ Arrhythm Electrophysiol 2014;7:532-42. [Crossref] [PubMed]
- Leenders GE, Lumens J, Cramer MJ, et al. Septal deformation patterns delineate mechanical dyssynchrony and regional differences in contractility: analysis of patient data using a computer model. Circ Heart Fail 2012;5:87-96. [Crossref] [PubMed]
- Motonaga KS, Miyake CY, Punn R, et al. Insights into dyssynchrony in hypoplastic left heart syndrome. Heart Rhythm 2012;9:2010-5. [Crossref] [PubMed]
- Kubuš P, Materna O, Tax P, et al. Successful permanent resynchronization for failing right ventricle after repair of tetralogy of Fallot. Circulation 2014;130:e186-90. [Crossref] [PubMed]
- Takayasu H, Takahashi K, Takigiku K, et al. Left ventricular torsion and strain in patients with repaired tetralogy of Fallot assessed by speckle tracking imaging. Echocardiography 2011;28:720-9. [Crossref] [PubMed]
- Menting ME, Eindhoven JA, van den Bosch AE, et al. Abnormal left ventricular rotation and twist in adults with corrected tetralogy of Fallot. Eur Heart J Cardiovasc Imaging 2014;15:566-74. [Crossref] [PubMed]
- Yim D, Riesenkampff E, Caro-Dominguez P, et al. Assessment of diffuse ventricular myocardial fibrosis using native T1 in children with repaired tetralogy of Fallot. Circ Cardiovasc Imaging 2017;10:e005695. [Crossref] [PubMed]
- Filippov AA, Del Nido PJ, Vasilyev NV. Management of systemic right ventricular failure in patients with congenitally corrected transposition of the great arteries. Circulation 2016;134:1293-302. [Crossref] [PubMed]
- Brida M, Diller GP, Gatzoulis MA. Systemic right ventricle in adults with congenital heart disease: anatomic and phenotypic spectrum and current approach to management. Circulation 2018;137:508-18. [Crossref] [PubMed]
- Diller GP, Okonko D, Uebing A, et al. Cardiac resynchronization therapy for adult congenital heart disease patients with a systemic right ventricle: analysis of feasibility and review of early experience. Europace 2006;8:267-72. [Crossref] [PubMed]
- Kalogeropoulos AP, Deka A, Border W, et al. Right ventricular function with standard and speckle-tracking echocardiography and clinical events in adults with D-transposition of the great arteries post atrial switch. J Am Soc Echocardiogr 2012;25:304-12. [Crossref] [PubMed]
- Diller GP, Radojevic J, Kempny A, et al. Systemic right ventricular longitudinal strain is reduced in adults with transposition of the great arteries, relates to subpulmonary ventricular function, and predicts adverse clinical outcome. Am Heart J 2012;163:859-66. [Crossref] [PubMed]
- Eindhoven JA, Menting ME, van den Bosch AE, et al. Quantitative assessment of systolic right ventricular function using myocardial deformation in patients with a systemic right ventricle. Eur Heart J Cardiovasc Imaging 2015;16:380-8. [Crossref] [PubMed]
- Bos JM, Hagler DJ, Silvilairat S, et al. Right ventricular function in asymptomatic individuals with a systemic right ventricle. J Am Soc Echocardiogr 2006;19:1033-7. [Crossref] [PubMed]
- Pettersen E, Helle-Valle T, Edvardsen T, et al. Contraction pattern of the systemic right ventricle: shift from longitudinal to circumferential shortening and absent global ventricular torsion. J Am Coll Cardiol 2007;49:2450-6. [Crossref] [PubMed]
- Becker M, Hümpel C, Ocklenburg C, et al. The right ventricular response to high afterload: comparison between healthy persons and patients with transposition of the great arteries: a 2D strain study. Echocardiography 2010;27:1256-62. [Crossref] [PubMed]
- Chow PC, Liang XC, Cheung YF. Diastolic ventricular interaction in patients after atrial switch for transposition of the great arteries: a speckle tracking echocardiographic study. Int J Cardiol 2011;152:28-34. [Crossref] [PubMed]
- Latus H, Hachmann P, Gummel K, et al. Impact of right ventricular outflow tract obstruction on biventricular strain and synchrony in patients after repair of tetralogy of Fallot: a cardiac magnetic resonance feature tracking study. Eur J Cardiothorac Surg 2015;48:83-90. [Crossref] [PubMed]
- Schranz D, Akintuerk H, Bailey L. Pulmonary artery banding for functional regeneration of end-stage dilated cardiomyopathy in young children: World Network Report. Circulation 2018;137:1410-2. [Crossref] [PubMed]
- Kowalik E, Mazurkiewicz L, Kowalski M, et al. Echocardiography vs magnetic resonance imaging in assessing ventricular function and systemic atrioventricular valve status in adults with congenitally corrected transposition of the great arteries. Echocardiography 2016;33:1697-702. [Crossref] [PubMed]
- Ladouceur M, Redheuil A, Soulat G, et al. Longitudinal strain of systemic right ventricle correlates with exercise capacity in adult with transposition of the great arteries after atrial switch. Int J Cardiol 2016;217:28-34. [Crossref] [PubMed]
- Chow PC, Liang XC, Lam WW, et al. Mechanical right ventricular dyssynchrony in patients after atrial switch operation for transposition of the great arteries. Am J Cardiol 2008;101:874-81. [Crossref] [PubMed]
- Forsha D, Risum N, Smith PB, et al. Frequent activation delay-induced mechanical dyssynchrony and dysfunction in the systemic right ventricle. J Am Soc Echocardiogr 2016;29:1074-83. [Crossref] [PubMed]
- Morcos M, Kilner PJ, Sahn DJ, et al. Comparison of systemic right ventricular function in transposition of the great arteries after atrial switch and congenitally corrected transposition of the great arteries. Int J Cardiovasc Imaging 2017;33:1993-2001. [Crossref] [PubMed]
- Iriart X, Roubertie F, Jalal Z, et al. Quantification of systemic right ventricle by echocardiography. Arch Cardiovasc Dis 2016;109:120-7. [Crossref] [PubMed]
- Kutty S, Li L, Polak A, et al. Echocardiographic knowledge-based reconstruction for quantification of the systemic right ventricle in young adults with repaired D-transposition of great arteries. Am J Cardiol 2012;109:881-8. [Crossref] [PubMed]
- de Leval MR, Kilner P, Gewillig M, et al. Total cavopulmonary connection: a logical alternative to atriopulmonary connection for complex Fontan operations. Experimental studies and early clinical experience. J Thorac Cardiovasc Surg 1988;96:682-95. [PubMed]
- d‘Udekem Y, Iyengar AJ, Galati JC, et al. Redefining expectations of long-term survival after the Fontan procedure: twenty-five years of follow-up from the entire population of Australia and New Zealand. Circulation 2014;130:S32-8. [Crossref] [PubMed]
- Udink Ten Cate FEA. Stenting the Fontan pathway in paediatric patients with obstructed extracardiac conduits. Heart 2017;103:1111-6. [Crossref] [PubMed]
- Gewillig M, Goldberg DJ. Failure of Fontan circulation. Heart Fail Clin 2014;10:105-16. [Crossref] [PubMed]
- Mori M, Aquirre AJ, Elder RW, et al. Beyond a broken heart: circulatory dysfunction in the failing Fontan. Pediatr Cardiol 2014;35:569-79. [Crossref] [PubMed]
- Rychik J, Goldberg D, Rand E, et al. End-organ consequences oft he Fontan operation: liver fibrosis, protein-losing enteropathy and plastic bronchitis. Cardiol Young 2013;23:831-40. [Crossref] [PubMed]
- Holler F, Hannes T, Germund I, et al. Low serum 25-hydroxyvitamin D levels and secondary hyperparathyroidism in Fontan patients. Cardiol Young 2016;26:876-84. [Crossref] [PubMed]
- Anderson PA, Sleeper LA, Mahony L, et al. Contemporary outcomes after the Fontan procedure: a Pediatric Heart Network multicenter study. J Am Coll Cardiol 2008;52:85-98. [Crossref] [PubMed]
- Khairy P, Fernandes SM, Mayer JE Jr, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation 2008;117:85-92. [Crossref] [PubMed]
- Poh CL, Zannino D, Weintraub RG, et al. Three decades later: the fate of the population of patients who underwent the atriopulmonary Fontan procedure. Int J Cardiol 2017;231:99-104. [Crossref] [PubMed]
- Schlangen J, Petko C, Hansen JH, et al. Two-dimensional global longitudinal strain rate is a preload independent index of systemic right ventricular contractility in hypoplastic left heart syndrome patients after Fontan operation. Circ Cardiovasc Imaging 2014;7:880-6. [Crossref] [PubMed]
- Saiki H, Eidem BW, Ohtani T, et al. Ventricular-arterial function and coupling in the adult Fontan circulation. J Am Heart Assoc 2016;5:e003887. [Crossref] [PubMed]
- Logoteta J, Ruppel C, Hansen JH, et al. Ventricular function and ventriculo-arterial coupling after palliation of hypoplastic left heart syndrome: a comparative study with Fontan patients with LV morphology. Int J Cardiol 2017;227:691-7. [Crossref] [PubMed]
- Kato A, Riesenkampff E, Yim D, et al. Pediatric Fontan patients are at risk for myocardial fibrotic remodeling and dysfunction. Int J Cardiol 2017;240:172-7. [Crossref] [PubMed]
- Bellsham-Revell HR, Simpson JM, Miller OI, et al. Subjective evaluation of right ventricular systolic function in hypoplastic left heart syndrome: how accurate is it? J Am Soc Echocardiogr 2013;26:52-6. [Crossref] [PubMed]
- Hu L, Sun A, Guo C, et al. Assessment of global and regional strain left ventricular in patients with preserved ejection fraction after Fontan operation using a tissue tracking technique. Int J Cardiovasc Imaging 2019;35:153-60. [PubMed]
- Singh GK, Cupps B, Pasque M, et al. Accuracy and reproducibility of strain by speckle tracking in pediatric subjects with normal heart and single ventricular physiology: a two-dimensional speckle-tracking echocardiography and magnetic resonance imaging correlative study. J Am Soc Echocardiogr 2010;23:1143-52. [Crossref] [PubMed]
- Koopman LP, Geerdink L, Bossers SSM, et al. Longitudinal myocardial deformation does not predict single ventricle ejection fraction assessed by cardiac magnetic resonance imaging in children with a total cavopulmonary connection. Pediatr Cardiol 2018;39:283-93. [Crossref] [PubMed]
- Khoo NS, Smallhorn JF, Kaneko S, et al. Novel insights into RV adaptation and function in hypoplastic left heart syndrome between the first stages of surgical palliation. JACC Cardiovasc Imaging 2011;4:128-37. [Crossref] [PubMed]
- Park PW, Atz AM, Taylor CL, et al. Speckle-tracking echocardiography improves pre-operative risk stratification before the total cavopulmonary connection. J Am Soc Echocardiogr 2017;30:478-84. [Crossref] [PubMed]
- Friedberg MK, Silverman NH, Dubin AM, et al. Right ventricular mechanical dyssynchrony in children with hypoplastic left heart syndrome. J Am Soc Echocardiogr 2007;20:1073-9. [Crossref] [PubMed]
- Rösner A, Khalapyan T, Dalen H, et al. Classic-pattern dyssynchrony in adolescents and adults with a Fontan circulation. J Am Soc Echocardiogr 2018;31:211-9. [Crossref] [PubMed]
- Petko C, Hansen JH, Scheewe J, et al. Comparison of longitudinal myocardial deformation and dyssynchrony in children with left and right ventricular morphology after the Fontan operation using two-dimensional speckle tracking. Congenit Heart Dis 2012;7:16-23. [Crossref] [PubMed]
- Materna O, Kubus P, Janousek J. Right ventricular resynchronization in a child with hypoplastic left heart syndrome. Heart Rhythm 2014;11:2303-5. [Crossref] [PubMed]
- Enomoto Y, Aoki M, Nakamura Y, et al. Successful Fontan completion after cardiac resynchronization therapy. Circulation 2012;125:e655-8. [Crossref] [PubMed]
- Zhong SW, Zhang YQ, Chen LJ, et al. Ventricular twisting and dyssynchrony in children with single left ventricle using three dimensional speckle tracking imaging after the Fontan operation. Echocardiography 2016;33:606-17. [Crossref] [PubMed]
- Takahashi K, Inage A, Rebeyka IM, et al. Real-time 3-dimensional echocardiography provides new insight into mechanisms of tricuspid valve regurgitation in patients with hypoplastic left heart syndrome. Circulation 2009;120:1091-8. [Crossref] [PubMed]
- Kutty S, Colen T, Thompson RB, et al. Tricuspid regurgitation in hypoplastic left heart syndrome: mechanistic insights from 3-dimensional echocardiography and relationship with outcomes. Circ Cardiovasc Imaging 2014;7:765-72. [Crossref] [PubMed]
- Samad MD, Wehner GJ, Arbabshirani MR, et al. Predicting deterioration of ventricular function in patients with repaired tetralogy of Fallot using machine learning. Eur Heart J Cardiovasc Imaging 2018;19:730-8. [Crossref] [PubMed]
- Sengupta PP, Huang YM, Bansal M, et al. Cognitive machine-learning algorithm for cardiac imaging. A pilot study for differentiating constrictive pericarditis from restrictive cardiomyopathy. Circ Cardiovasc Imaging 2016;9:e004330. [Crossref] [PubMed]
- Narula S, Shameer K, Salem Omar AM, et al. Machine-learning algorithms to automate morphological and functional assessments in 2D echocardiography. J Am Coll Cardiol 2016;68:2287-95. [Crossref] [PubMed]
- Cikes M, Tong L, Sutherland GR, et al. Ultrafast cardiac ultrasound imaging: technical principles, applications, and clinical benefits. JACC Cardiovasc Imaging 2014;7:812-23. [Crossref] [PubMed]
- Joos P, Poree J, Liebgott H, et al. High-frame rate speckle tracking echocardiography. IEEE Trans Ultrason Ferroelectr Freq Control 2018;65:720-8. [Crossref] [PubMed]


