
Hypertrophic cardiomyopathy: genetics and clinical perspectives
Prevalence and definition
Hypertrophic cardiomyopathy (HCM) is the most common inherited heart disease with a prevalence of 1/200 (1) to 1/500 (2).
The disease was first described as “Idiopathic subaortic stenosis” 60 years ago (3) and later classified as “hypertrophic cardiomyopathy” with or without left ventricular outflow tract (LVOT) obstruction based on functional and morphologic features by the European guidelines (4) and based on etiology by guidelines of the American Heart Association (5).
During the past two decades, mutations in genes that encode for proteins of the contractile apparatus of the cardiomyocyte have been identified as cause of the disease (6-8). Since then, over 450 mutations in 20 sarcomeric and myofilament-related proteins have been identified for HCM (5,7,9). However, most mutations affect genes encoding for the β-myosin heavy chain (MYH7) and myosin binding protein C (MYBPC3) of the cardiac sarcomere (9).
HCM is characterized by isolated progressive myocardial hypertrophy in the absence of another cardiac or systemic disease, typical histopathologic changes such as fibrosis and myocyte disarray, systolic and diastolic ventricular dysfunction, and arrhythmias (5,10). HCM is the number one reason for sudden cardiac death (11) in young adulthood (12,13) and in young athletes (14,15), but optimized risk stratification, prohibition of competitive sports, and the implantation of cardioverter-defibrillators (ICD) has dramatically lowered mortality within the last decade (16).
With contemporary multidisciplinary and evidence-guided management life expectancy is usually relatively favorable (17-19). Approximately two thirds of patients with HCM experience a normal life span without significant morbidity (20,21). In the remaining patients, atrial fibrillation, heart failure and stroke contribute to morbidity later in life (13,22), requiring symptomatic heart failure therapies and heart transplantation (23,24).
Secondary causes of left ventricular hypertrophy (LVH), such as arterial hypertension, structural heart disease, or drug toxicity need to be ruled out before making the diagnosis of HCM. Other multisystem diseases, such as metabolic, endocrinologic, neuromuscular, neurologic, or autoimmune disorders and genetic syndromes can go along with typical features of HCM and need to be considered because management differs according to disease etiology (19,25-31) (Table 1, Figure 1). Those diseases should be termed disease-specific, such as Noonan cardiomyopathy or glycogen-storage cardiomyopathy to avoid confusion. The term “hypertrophic cardiomyopathy” or “HCM” should be used for disease secondary to mutations in the sarcomere or related structural proteins in the cardiomyocyte, or after careful exclusion of all systemic syndromes associated LVH mimicking HCM (10). The prevalence of HCM has become greater with the use of more sensitive imaging methods and the advance genetic testing allowing a molecular diagnosis prior to the onset of ventricular hypertrophy, which is an age-dependent process (1,2,33-35).
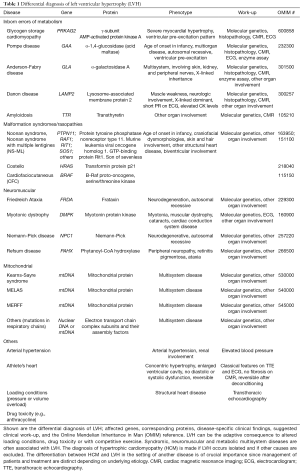
Full table
Pathogenesis
HCM is caused by mutations in sarcomere genes
The underlying genetic cause of HCM was described in the 1990 by identification of a sarcomere mutation in a large family presenting with HCM, sudden death, and heart failure (6,36). Since then, the report of multiple separate mutations in distinct sarcomere proteins (37), the regulators of contraction and relaxation of the heart, established HCM as a genetically heterogeneous disease. Among the known causal genes, MYH7 and MYBPC are the two most common (38,39) followed by mutations in TNNT2 and TNNI3 (40,41). Rarely reported are mutations in genes encoding for other components of the sarcomere (42) (Table 2).
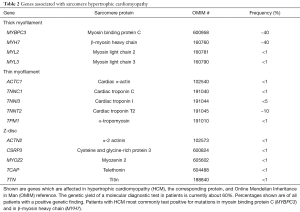
Full table
Disease variability in HCM
Carrying a heterozygous sarcomere gene mutation alone cannot fully explain HCM pathology as the clinical course of the disease varies greatly with variable expressivity (8), age penetrance, and a high clinical heterogeneity, even within patients or family members carrying the same mutation (43-46) or within genetically engineered identical mice (47).
Thus, the primary defect is the sarcomere mutation, but clinical expression is determined by a complex hierarchy of genetic, epigenetic, and environmental factors (Figure 2).
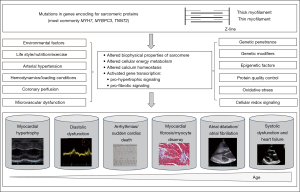
In the past decades, basic science and research on genetically modulated animal models (48), human tissue (49) and human induced pluripotent cell models (50) contributed to our understanding of disease.
First, mutations directly alter the structure and function of the sarcomere proteins and alter biophysical properties of the cardiomyocyte (51-56), influence calcium handling (57), and change cellular energy balance (58-61). Additionally, mutations can directly initiate other signaling pathways via transcriptional activation (62,63); including expression and activation of trophic and mitotic factors, such as calcineurin, mitogen-activated protein kinases, and transforming growth factor beta pathways (64-67) and stimulate non-cardiac cells, such as fibroblasts (68). Genetic variants in signaling pathways implicated in regulating cardiac hypertrophy and fibrosis can influence expression of the HCM phenotype and function as modified genetic variants. Hence, modifier variants due to distinct genetic background can in part, explain interindividual variability in the phenotypic expression of HCM (69).
In addition to direct effects of the underlying sarcomere mutation, secondary molecular and intracellular changes occur in response to the changes of sarcomere protein structure and function. Those include epigenetic modifications and posttranslational modifications, such as micro RNAs (miRNAs), small noncoding RNAs with 22 nucleotides, regulating gene expression at the posttranscriptional level (70,71) and histone modifications (57,65,68,72).
The primary and secondary effects of the mutations ultimately lead to the functional pathological phenotypes, such as myocardial hypertrophy and ventricular dysfunction. The interplay of microvascular ischemia, cardiomyocyte energy depletion and apoptosis, leads to adverse remodeling with progressive myocyte loss and fibrous substitution of the myocardium, presenting as the histological phenotypes of myocyte disarray and myocardial fibrosis (60,73-75). The same pathomechanisms that cause adverse remodeling ultimately lead to the irreversible stage of overt dysfunction and ultimately severe heart failure and death (76,77).
Arrhythmias in HCM are caused by both the primary mutation effects, such as disturbed calcium handling causing triggered activity by electrical afterdepolarization (78), as well as the secondary effects, such as cardiomyocyte hypertrophy increasing myocyte automaticity (79) and myocardial fibrosis creating reentry (80-83) (Figure 3). Transient pathophysiological factors, such as maladaptive autonomic responses, myocardial ischemia, and altered hemodynamics (81,84), influence arrhythmogenicity in HCM.
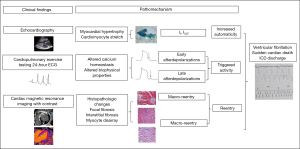
Clinical disease stages and underlying myocardial pathology
The pathogenic HCM mutation initiates a life-long remodeling process within the myocardium which presents with distinct clinical disease stages (60,85,86). Initially, there is the “non-hypertrophic HCM” which is characterized by the absence of LV hypertrophy in individuals harboring HCM-causing mutations, investigated during systemic family screening (stage 0, Figure 4). The prevalence of patients in this stage has increased given incorporation of systemic family screening and molecular testing and is estimated at about 0.6% (1:167) (1). Individuals in this stage are defined as “genotype-positive/phenotype-negative” (Genotype +/Phenotype−). Medical notes should describe such patients’ diagnosis as “pre-clinical hypertrophic cardiomyopathy”, or “hypertrophic cardiomyopathy without clinical signs”.
Stage I
Stage I of the disease is defined by the development of LVH with or without LVOT obstruction, hyperdynamic ventricular function, and mild symptoms, such as decreased exercise tolerance or intermittent chest pain. Arrhythmias can occur during this stage (stage I, Figure 4). Onset of this stage occurs in approximately one half of patients by the third decade of life and in about three fourths by the sixth decade (59,60,85,87). Subtle abnormalities on electrocardiogram (ECG) or on transthoracic echocardiography (TTE), such as impaired LV relaxation, mitral valve abnormalities or mild left atrial dilatation might be present during this stage. Elevated levels of type I collagen precursors (88) and altered coronary microvascular function (74) have been described in this stage. Counseling regarding prognosis in this stage is challenging with current methodologies. Risk for sudden cardiac death is generally low, but potentially malignant arrhythmias can occur, specifically in patients harboring a mutation in the cardiac troponin T gene (89).
Stage II
Adverse myocardial remodeling with increasing myocardial hypertrophy and fibrotic changes occurs during stage II of the disease (stage II, Figure 4). The prevalence of this stage is estimated at about 15% (85). Increasing LV fibrosis and worsening function with relatively preserved clinical and hemodynamic balance occurs during this stage. The extent and the time-course of LV remodeling are extremely heterogeneous (90). The clinical findings in this stage are a low to normal range LV ejection fraction (91), moderate to severe diastolic dysfunction (92), atrial dilatation (93), areas of late gadolinium enhancement (91), microvascular dysfunction (74), thinning of LV walls (94), onset of atrial fibrillation (95), and LV apical aneurysms (96). The clinical correlates of adverse remodeling may vary widely, ranging from mild to severe manifestations. Congestive symptoms may become evident by impairment of cardiopulmonary exercise testing (CPET) and elevated titers of natriuretic peptides (97).
Stage III
Stage III presents then the irreversible “end-stage” of disease with high morbidity and mortality. The stage is characterized by extreme degrees of LV fibrosis, progression to LV dilatation, atrial dilatation, and systolic and diastolic dysfunction associated with hemodynamic decompensation, heart failure-related complications, heart transplantation or death (90,98). A small number of HCM patients (<5%) progress to this end-stage phase of HCM (85,99). The stage can be differentiated between the so called “hypokinetic-dilated” form, characterized by a decrease in left ventricular wall thickness while end-diastolic cavity dimensions increases and ventricular function deteriorates (94), and the so called “hypokinetic-restrictive” form, characterized by a small and stiff LV with extreme diastolic dysfunction, resembling primary restrictive cardiomyopathy. In this form, systolic function is only mildly or moderately impaired, but marked bilateral atrial dilatation and atrial fibrillation are usually present (100). This form has been associated with mutations in thin filaments (101) but also occurs with other sarcomere mutations.
The extent and the rate at which each of these features occur and evolve are very variable determining clinical heterogeneity. In most of the patients, disease onset occurs between 20 and 50 years of age (22), but some patients also present during childhood (102), while others remain asymptomatic until late in life (85,103).
Clinical work-up
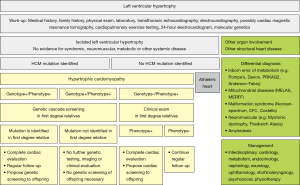
Once the suspicion of HCM has been made, or once a family member has been diagnosed with HCM, specific work-up is required for the presenting individual (Figure 1). Thorough clinical work-up including a detailed medical and family history, general and focused physical examination, electrophysiological assessment by electrocardiogram (ECG), 24-hour ECG, CPET, imaging by transthoracic echocardiography (TTE) and cardiovascular magnetic resonance imaging (CMR), as well as general and molecular genetic testing (Table 3) is required to exclude concomitant conditions that may mimic HCM (Table 1) and to appropriately stage HCM (Figure 4).
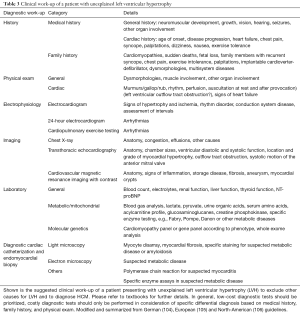
Full table
Importance of medical and family history
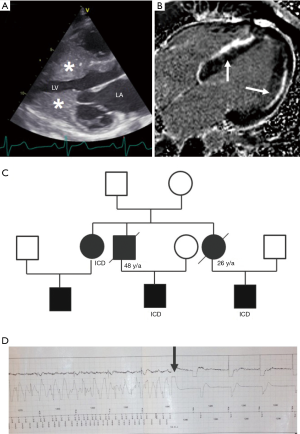
Despite the presence of cardiac hypertrophy, patients with HCM are commonly asymptomatic or minimally symptomatic. Often HCM is diagnosed in asymptomatic patients by the incidental finding of a murmur or by a family member being diagnosed with HCM and prompting family evaluation. Approximately 60% of patients with HCM have a clearly recognizable familial disease (107). A detailed family history and generation of a family tree (108) is helpful in evaluating phenocopy conditions mimicking HCM (109), inheritance pattern and malignancy of HCM phenotype, and identifying other family members at risk (110-114) (Figure 5). In HCM there is usually an autosomal dominant pattern of inheritance (115).
Assessment of arrhythmias and electrocardiographic parameters
Rhythm disturbances in HCM include the occurrence of supraventricular and ventricular ectopic beats, and rarely with non-sustained or sustained ventricular tachycardia (116-118). Atrial fibrillation occurs in patients with advanced clinical stage or severe LVOT obstruction (119,120) and is a major risk factor for heart failure (95) and for thromboembolic stroke (121). Left atrial size and function as well as LVOT obstruction are major risk factors for atrial fibrillation.
Arrhythmias can be detected by ECG, 24-hour ECG (122), or CPET (123).
In general, ECG is abnormal in most patients with HCM and may show voltage changes of LVH, ST-T wave changes, T-wave inversions, pathological deep Q waves caused by depolarization of a hypertrophied interventricular septum, complete bundle branch block and evidence of left atrial enlargement (124). Extremely high-voltage QRS complexes or a delta-wave should raise the possibility of a phenocopy condition mimicking HCM. Common ECG patterns in athletes might include sinus bradycardia, sinus arrhythmia, J-point elevation with ascending ST-segments, first degree atrioventricular block, and incomplete right bundle branch block (125). Peak oxygen consumption on CPET can help to differentiate between athlete’s heart and HCM, being high in athletes and low in HCM.
Imaging in HCM
Imaging in HCM is imperative to establish the diagnosis, risk stratification, and disease monitoring of patients (126,127).
Echocardiographic examination by TTE is the primary imaging modality used in evaluation of a patient to exclude structural heart disease, and to assess myocardial morphology and function.
LVH on TTE in an adult is defined by a wall thickness of equal or greater than 15 mm in one or more left ventricular myocardial segments and equal or greater than 13 mm in first-degree relatives of HCM patients (2,4,32) (Figure 5). In children, cardiac hypertrophy is defined by z-scores above 2 of the age- and sex-matched population (128). Hypertrophy in HCM is commonly asymmetrical with the most severe hypertrophy involving the basal interventricular septum (129). Occasionally, myocardial hypertrophy is restricted to other myocardial regions, such as the apex, the midportion, and the posterior wall of the left ventricle (130). Extreme ventricular hypertrophy or the involvement of the right ventricle should prompt the possibility of a phenocopy condition mimicking HCM, although rarely the right ventricle can be involved in HCM (85,131). Physiological cardiac hypertrophy in adolescents and adults who are active in competitive sports exhibits with left ventricular wall thickness between 13 and 18 mm (132). A distinguishing feature is a symmetrical enlargement of all cardiac chambers (133), and the size of the left ventricular cavity, which is not enlarged in HCM, but is typically enlarged in the physiological hypertrophy of the athlete’s heart (134,135). In addition, the distribution pattern of cardiac hypertrophy with asymmetrical septal hypertrophy strongly favors HCM (136). The electrical and structural changes in athletes are considered benign and generally reversible after detraining.
Left ventricular outflow tract obstruction is present at rest or provoked in about two thirds of patients with HCM (21,25,107,137,138). Systolic anterior motion of the anterior mitral valve (SAM) might contribute to LVOT obstruction (3). Other frequent pathological features include elongation of the anterior or both leaflets of the mitral valve, as well as abnormal insertion of the associated papillary muscles (139).
Diastolic dysfunction in HCM is frequently detected by atrial enlargement and by abnormal pulsed Doppler tissue imaging on TTE (121,140-142).
Ejection fraction is elevated during the first clinical stages and reduced in end-stage HCM.
CMR is often necessary to exclude phenocopy conditions, such as Anderson-Fabry disease, amyloidosis, or other storage diseases (143). Additionally, morphological abnormalities, including myocardial crypts, left ventricular aneurysms or focal myocardial hypertrophy can be detected best by CMR (1,129,144,145). Focal fibrotic changes are detected by positive late gadolinium enhancement (2,88,146-148) (Figure 5) and interstitial fibrosis by increased extracellular volume fraction on CMR T1 map (149). Contrast CMR can help in identifying patients that transition from clinical stages I to clinical stage II of adverse remodeling (91), but the advisable frequency of scans during follow-up remains to be determined (18).
Other more advanced imaging technologies, such as CMR diffusion tensor imaging to assess myofibrillar orientation (150,151), single-photon emission computed tomography (PET) (127), or the use of speckle tracking and strain imaging on echocardiography and CMR (152-156) are under investigation but not in routine clinical use yet.
Laboratory and molecular genetic testing
General laboratory examination and specific metabolic testing, such as enzyme-assays to detect specific glycogen or lysosomal storage disorders, needs to be undertaken to exclude phenocopy conditions mimicking HCM (157).
Molecular testing has now become a powerful diagnostic aid in routine cardiovascular practice through commercially available DNA-based testing for disease-causing mutations (158,159) and can help to diagnose both HCM as well as phenocopy conditions (Table 1).
Genetic testing is a class I indication in both the European as well as the North-American guidelines (32,106,107,160). Molecular genetic testing is indicated in patients with clinical signs and symptoms for HCM suggestive of HCM to confirm diagnosis, in patients fulfilling HCM diagnosis to enable genetic cascade screening in relatives, and in first degree relatives with definite disease-causing mutations after pre-test counseling (Figure 1).
Genetic testing, when positive, will support the diagnosis in a patient, but if it is negative, it will not exclude it.
The majority of genes and mutations, which are responsible for clinically diagnosed HCM, encode proteins of the sarcomere (6,19,161) (Table 2). Of those, MYH7 and MYBPC3, encoding β-myosin heavy chain and myosin-binding protein C, respectively, are the two most common genes involved, together accounting for about 50% of the HCM families. In about 40% of HCM patients, the causal genes remain to be identified (32,106,107,162).
Criteria for assessing variant pathogenicity are based on the variant type, variant database, literature review, frequency in the general population, and in silico analysis, meaning the influence of the genetic variant on protein morphology and function (163,164). However, interpretation of the findings in molecular genetic testing can be extremely challenging. Each human genome contains an average of about 11,000 nonsynonymous variants, 160 premature protein truncation variants, and 500,000 variants in the known regulatory regions (165). Given variability of phenotypic expression of HCM there is often uncertain evidence of causality for many mutations possibly implicated in HCM (39,42). This can result in reclassification of variants (166,167).
Cardiac catheterization and endomyocardial biopsy
Given modern imaging modalities and molecular genetic testing as well as enzyme testing, cardiac catheterization and endomyocardial biopsy is not performed in the first line of diagnosis in a patient presenting with LVH. However, in unresolved or unclear conditions, endomyocardial biopsy and specific histological examination may identify phenocopy conditions mimicking HCM, such as glycogen-filled cardiomyocytes in glycogen-storage cardiomyopathy (157) or amyloid depositions in amyloidosis (168). In HCM, histopathology of endomyocardial biopsy specimens with identify enlarged cardiac myocytes that present in disarray with loss of normal parallel alignment giving a swirling appearance to the myocardial architecture (169-171). Additionally, focal and interstitial fibrotic replacement of the myocardium on histopathology is pathognomic for HCM (9).
Management
Phenocopy conditions mimicking HCM need to be diagnosed in time because of the possibility of medical treatment and cure in a subset of those diseases by enzyme replacement therapies specifically in Anderson-Fabry (26-29) and in Pompe’s disease (30) or by supportive therapies (e.g., Co-enzyme Q10 in mitochondriopathies) (31).
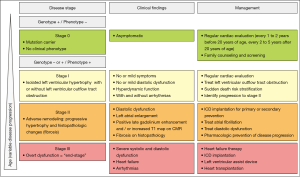
No causal therapy is available for HCM, so that the focus of current management is on early identification of asymptomatic patients at risk through molecular diagnostic and clinical cascade screening of family members, optimal sudden death risk stratification, and timely initiation of preventative therapies (94,172) to avoid disease progression to the irreversible adverse myocardial remodeling stage (17,76) (Figure 4).
During stage 0 of disease, there is a focus on appropriate counseling of patients and families about the disease pathogenesis and basic understanding of the genetic and molecular basis of the disease should be done during this stage. This is of importance, since asymptomatic patients might otherwise be lost to follow-up and suffer from sudden arrhythmic death or advance to the severe adverse remodeling stage without timely prevention strategies. If not already done, patients should also be referred to specialized centers with interdisciplinary teams consisting of cardiologist, pediatric cardiologist and human geneticist (173).
Relief of LVOT obstruction (19), avoiding competitive sports and risk stratification for sudden death is the mainstay of therapy during stage I of the disease. Control of conventional risk factors such as sedentary lifestyle, hypertension, dyslipidemia, and diabetes (174), and regular clinical follow-ups to timely identify progression to stage II are necessary during stage I (Figure 4, Table 4).
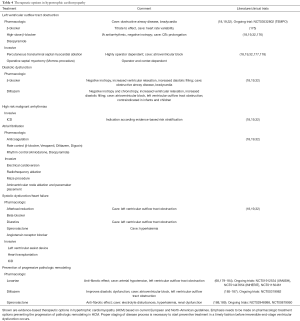
Full table
HCM patients in stage II with adverse cardiac remodeling are considered at risk of further progression toward overt dysfunction and need close clinical surveillance with CMR, CPET, serial NT-proBNP assessment to allow for preventive treatment (190). The decline in LV function and progression of symptoms may be slow and patients can be stabilized for years by heart failure treatment (18,19,59). Patients with the hypokinetic-restrictive subtype are more challenging to manage as they do not tolerate or benefit from standard heart failure therapies. In those patients it is crucial to avoid missing a window for transplant listing (98). Aggressive treatment of atrial fibrillation might play a role during this stage to prevent functional and clinical deterioration in HCM patients (95).
Management of overt dysfunction in stage III is based on standard guidelines for heart failure (18) (Figure 4, Table 4). Commonly accepted measures include ACE inhibitors and angiotensin receptor blockers, heart-failure specific β-blockers, spironolactone, loop diuretics, and, in the presence of atrial fibrillation or an apical aneurysm, oral anticoagulants for cardioembolic prevention (18,19,86,90,95). In addition, overt dysfunction should be considered as potential indication for primary arrhythmic death and implantable cardioverter-defibrillator (ICD) implantation (90). Resynchronization by biventricular pacing may represent a promising option for the improvement of LV efficiency and symptoms in HCM patients with overt dysfunction (191). Tailored surgical options may present in individual cases, such as mitral plasty to correct annulus dilation and regurgitation, implantation of an left ventricular assist device, and cardiac transplantation (77,98).
Family evaluation
Evaluation of family members by clinical and molecular genetic screening is a class I indication in both the European and North-American guidelines (32,106,107,160) (Figure 1). If a disease-causing mutation has been identified, specific genetic testing can be performed in offspring (104,106). If no mutation has been identified, clinical examination needs to be undertaken every 3 to 5 years before 10 years of age, every 1 to 2 years between 10 and 20 years of age, and every 2 to 5 years after 20 years of age since HCM has an age-dependent disease onset and disease cannot be excluded until later in life (45,160).
Prevention and sudden death risk stratification
Given the high risk for sudden cardiac death in competitive athletes (14,15) and the possibility of disease acceleration with exercise (48,192,193), European and North-American guidelines do not recommend the participation in competitive sports for patients with clinically overt HCM (stage I and above) (32,194). There is an ongoing debate whether participation in competitive sports is allowed in genotype positive/phenotype negative patients, as it is according to North-American guidelines (18), or whether no competitive sports are allowed in those patients as proposed by European guidelines (32,195).
The major cause of sudden cardiac death in HCM is ventricular fibrillation and treatment with an ICD is the only preventive measure recommended in high risk patients.
According to the European (19,32) and North-American guidelines (18,19), an ICD is recommended as secondary prevention in HCM patients who have survived a cardiac arrest caused by ventricular fibrillation or sustained ventricular tachycardia (196,197).
For primary prevention, ICD indication is based on multiple clinical factors identified as risk factors for malignant arrhythmias and sudden cardiac death by large studies (198-201). Those risk factors include massive LV hypertrophy (>30 mm or z-score >6) (122,196,202,203), syncope of unknown etiology (204), family history of sudden death <40 y/age (205-207), non-sustained ventricular tachycardia (122,196,205,207), and abnormal blood pressure response on stress test (196). An online available risk prediction model for sudden cardiac death in HCM (
Pharmacotherapy
For a patient with HCM but no symptoms, no drugs are recommended by current ACC and ESC guidelines (19).
In the presence of LVOT obstruction, diastolic dysfunction and heart failure, β-blockers are the most commonly used pharmacological agents and the first choice in the absence of a contraindication (Table 4). The proposed mechanisms of effects include improved ventricular relaxation and increased diastolic filling time and, hence, improved left ventricular end diastolic pressure as well as perfusion (175,218). The benefits of β-blockers on mortality and the risk of SCD in patients with HCM and their impact on prevention or reversal of cardiac hypertrophy in HCM remains to be established.
Disopyramide is a negative inotropic agent and may reduce symptoms further in patients with LVOTO when added to β-blockers in patients with LVOT obstruction (176) (Table 4). However, this medication is not available in all countries.
L-type calcium channel blockers, such as verapamil or the non-dihydropyridine calcium channel blocker diltiazem, may be beneficial in patients who do not tolerate or respond to β-blockers (219), but they are contraindicated in patients with LVOT obstruction and in children. Verapamil and diltiazem exert their beneficial effects in part through their negative inotropic and chronotropic effects and in part through improving myocardial diastolic properties (Table 4).
Diuretics may be used in patients with HCM, pulmonary congestion, and heart failure, but minimal effective doses and careful observation are required to avoid hypovolemia, hypotension, and intensification or provocation of LVOT obstruction (Table 4).
Long-term anticoagulation is necessary in patients with persistent and paroxysmal atrial fibrillation because of risk for thromboembolism (121) (Table 4).
The conventionally used pharmacological agents in treatment of patients with HCM have not been shown to reverse or attenuate established cardiac hypertrophy and fibrosis, although adverse remodeling is a major determinant of mortality and morbidity in patients with HCM. Thus, effective treatment of HCM needs to target the molecular mechanisms that are involved in the pathogenesis of the phenotype. Pharmacological strategies aimed at preventing development of LV hypertrophy and adverse remodeling stage have been proposed, based on encouraging preclinical data with agents such as statins, losartan, diltiazem and others (68,179,220-223) (Table 4). Studies in animal models of HCM suggest possible prevention of progression to adverse remodeling stage with angiotensin I receptor blockers (68,179), calcium channel blockers, statins (220,221), mineralocorticoid receptor blockers (222), and antioxidant N-acetylcysteine (223). Although some preliminary studies in humans suggested a similar effect of those therapies in humans (180), data at this point are contradictory (180,181,224) and multiple clinical trials are currently ongoing (182,225) (Table 4). Specific treatments targeting cardiomyocyte energy deficiency, microvascular dysfunction and the development of fibrosis are currently being investigated (190). RNA based therapies in HCM have so far been shown to be efficient in mouse models (226) and in several in vivo studies (227), but no clinical trials have been undertaken so far due to delivery system and safety problems (228).
A promising new agent might be MYK-461, an orally administered small molecule that allosterically inhibits myosin ATPase activity and diminishes myocyte force production. MYK-461 has been shown to suppress development of cardiac hypertrophy, myocyte disarray, and fibrosis in a mouse model of HCM (52). After successful completion of 3 phase I clinical trials with this compound, it is now being evaluated in a phase II clinical trial in patients with HCM and LVOT obstruction (NCT02842242).
Care needs to be taken since any medication that may cause peripheral vasodilation and afterload reduction (e.g., ACE-inhibitors, angiotensin-I receptor blockers, dihydropyridine calcium channel blockers, nitrates), intravascular volume depletion (e.g., diuretics), or positive inotropy (e.g., digoxin or inotropic agents) can worsen the LV-outflow tract obstruction and should be avoided in patients with HCM and obstructive physiology.
Invasive management
The consensus document recommends that patients with drug refractory LVOT obstruction (systolic pressure gradient of equal or greater than 50 mmHg at rest or with provocation) should be considered for septal reduction therapy, either surgical septal myectomy or alcoholic septal ablation (19) (Table 4). Both techniques of septal reduction therapy are highly operator dependent. The final decision as to which approach should be selected in any given patient is dependent up patient preference and the availability and experience of the operator and institution at which the patient is being treated.
Left ventricular assist devices and heart transplantation are necessary in end-stage HCM patients with drug-refractory heart failure (229). Although post-transplant survival in HCM is excellent, waitlist mortality remains substantial (230,231).
Surgical septal myectomy
Surgical septal myectomy (177) is the gold-standard for the relief of drug-refractory symptoms in patients with obstructive HCM (32,178) and improves survival, exercise capacity and quality of life in those patients (232,233). When performed by experienced operators working in high-volume centers, septal myectomy by a transaortic approach is highly effective with low perioperative mortality rate (234). Midventricular or apical myectomy is rarely performed by the transapical approach for patients with HCM variants and refractory heart failure symptoms with the purpose to increase the left ventricular end diastolic and systolic dimensions resulting in an increase in stroke volume (235). Left ventricular outflow obstruction is often aggravated by an abnormal mitral valve and subvalvular apparatus (236). In most patients mitral regurgitation related to systolic anterior motion of the mitral valve is relieved through adequate myectomy with or without papillary muscle reduction (237). If intrinsic mitral valve disease is present, mitral valve plasty should be the first-line treatment preferred over mitral valve replacement (238,239).
Alcohol septal ablation
Catheter-based alcohol septal ablation to create a septal infarction has emerged as a less invasive percutaneous alternative to surgical septal myectomy. The results of alcohol septal ablation are dependent on the septal perforator artery supplying the area of the contact between the hypertrophied septum and the anterior leaflet of the mitral valve. A transradial approach has been shown to be associated with fewer access-related complications compared to the classical femoral artery access (240,241). There is a substantial risk for developing complete atrioventricular block requiring pacemaker implantation after performing alcohol septal ablation (242) and the literature tends to support better long-term symptom relief in those patients who undergo surgical septal myectomy (243,244). At this point, young patients and patients requiring repair of associated abnormalities of the mitral valve and/or anomalous papillary muscles are not good candidates for alcohol septal ablation (245).
Biomarkers
Since preventative treatment is necessary to avoid disease progression in HCM patients, clinical disease stages need to be diagnosed in a timely fashion. The clinical utilities of plasma biomarkers in evaluation of cardiac hypertrophy and myocardial fibrosis in HCM remain to be established. However, there is evidence for cleavage products of collagen synthesis and degradation, as well as elevated levels of cytokines, cardiac troponin T, and other markers of myocardial inflammation as biomarkers for interstitial fibrosis (88,246-248). Circulating levels of several microRNAs (especially miR29a) are elevated in HCM (72,249) and may serve as markers for cardiac hypertrophy and interstitial fibrosis (250,251).
Summary
HCM is a highly prevalent disease and genetic discoveries have contributed to our understanding of the molecular disease pathogenesis.
Molecular diagnostics and new imaging modalities have optimized patient management. Mortality in HCM has significantly decreased by applying current standard therapeutic measures, such as the prevention of sudden death by prohibition of competitive sport participation and the implantation of cardioverter-defibrillators if indicated, as well as symptomatic heart failure therapies or cardiac transplantation.
However, therapies at this point are solely supportive and there is a lack of preventive or curative therapeutics.
In the future it is anticipated that the field will shift from targeting phenotypes, such as myocyte hypertrophy, fibrosis, arrhythmias, and LVOT obstruction, toward altering the underlying molecular pathways to prevent adverse remodeling disease stage. The timing of initiation of those preventive therapies is crucial because greatest potential occurs before conversion to end-stage disease. Modern imaging technologies and identification of new biomarkers will help in identifying progression of disease stages.
It is hoped that insights from clinical studies and basic science research will allow a shift from general approaches to tailored management according to individual needs in patients with HCM.
Acknowledgments
The author (Wolf CM) is supported by grants from the Stiftung Kinderherz, Deutsche Herzstiftung, Deutsche Zentrum für Herz-Kreislauf-Forschung (DZHK), and the Else Kroener-Fresenius-Foundation.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Semsarian C, Ingles J, Maron MS, et al. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol 2015;65:1249-54. [Crossref] [PubMed]
- Maron BJ, Gardin JM, Flack JM, et al. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation 1995;92:785-9. [Crossref] [PubMed]
- Braunwald E, Lambrew CT, Rockoff SD, et al. Idiopathic Hypertrophic Subaortic Stenosis. I. A Description of the Disease Based Upon an Analysis of 64 Patients. Circulation 1964;30 SUPPL 4:4-119. [PubMed]
- Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2008;29:270-6. [Crossref] [PubMed]
- Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006;113:1807-16. [Crossref] [PubMed]
- Seidman JG, Seidman C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell 2001;104:557-67. [Crossref] [PubMed]
- Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet 2013;381:242-55. [Crossref] [PubMed]
- Geisterfer-Lowrance AA, Kass S, Tanigawa G, et al. A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell 1990;62:999-1006. [Crossref] [PubMed]
- Seidman CE, Seidman JG. Identifying sarcomere gene mutations in hypertrophic cardiomyopathy: a personal history. Circ Res 2011;108:743-50. [Crossref] [PubMed]
- Maron BJ, Seidman CE, Ackerman MJ, et al. How should hypertrophic cardiomyopathy be classified?: What's in a name? Dilemmas in nomenclature characterizing hypertrophic cardiomyopathy and left ventricular hypertrophy. Circ Cardiovasc Genet 2009;2:81-5; discussion 86. [Crossref] [PubMed]
- Maron BJ. Hypertrophic cardiomyopathy. Lancet 1997;350:127-33. [Crossref] [PubMed]
- Maron BJ, Haas TS, Ahluwalia A, et al. Demographics and Epidemiology of Sudden Deaths in Young Competitive Athletes: From the United States National Registry. Am J Med 2016;129:1170-7. [Crossref] [PubMed]
- Ostman-Smith I, Wettrell G, Keeton B, et al. Age- and gender-specific mortality rates in childhood hypertrophic cardiomyopathy. Eur Heart J 2008;29:1160-7. [Crossref] [PubMed]
- Maron BJ. Contemporary insights and strategies for risk stratification and prevention of sudden death in hypertrophic cardiomyopathy. Circulation 2010;121:445-56. [Crossref] [PubMed]
- Watkins H. Sudden death in hypertrophic cardiomyopathy. N Engl J Med 2000;342:422-4. [Crossref] [PubMed]
- Maron BJ, Rowin EJ, Casey SA, et al. Hypertrophic Cardiomyopathy in Children, Adolescents, and Young Adults Associated With Low Cardiovascular Mortality With Contemporary Management Strategies. Circulation 2016;133:62-73. [Crossref] [PubMed]
- Olivotto I, Girolami F, Nistri S, et al. The many faces of hypertrophic cardiomyopathy: from developmental biology to clinical practice. J Cardiovasc Transl Res 2009;2:349-67. [Crossref] [PubMed]
- Gersh BJ, Maron BJ, Bonow RO, et al. ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2011;58:2703-38. [Crossref] [PubMed]
- Maron BJ, McKenna WJ, Danielson GK, et al. American College of Cardiology/European Society of Cardiology Clinical Expert Consensus Document on Hypertrophic Cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. Eur Heart J 2003;24:1965-91. [Crossref] [PubMed]
- Maron BJ, Rowin EJ, Casey SA, et al. Hypertrophic Cardiomyopathy in Adulthood Associated With Low Cardiovascular Mortality With Contemporary Management Strategies. J Am Coll Cardiol 2015;65:1915-28. [Crossref] [PubMed]
- Maron MS, Rowin EJ, Olivotto I, et al. Contemporary Natural History and Management of Nonobstructive Hypertrophic Cardiomyopathy. J Am Coll Cardiol 2016;67:1399-409. [Crossref] [PubMed]
- Maron BJ, Olivotto I, Spirito P, et al. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation 2000;102:858-64. [Crossref] [PubMed]
- Maron BJ, McKenna WJ, Danielson GK, et al. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol 2003;42:1687-713. [Crossref] [PubMed]
- Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011;124:e783-831. [PubMed]
- Maron BJ, Maron MS, Wigle ED, et al. The 50-year history, controversy, and clinical implications of left ventricular outflow tract obstruction in hypertrophic cardiomyopathy from idiopathic hypertrophic subaortic stenosis to hypertrophic cardiomyopathy: from idiopathic hypertrophic subaortic stenosis to hypertrophic cardiomyopathy. J Am Coll Cardiol 2009;54:191-200. [Crossref] [PubMed]
- Kampmann C, Wiethoff CM, Perrot A, et al. The heart in Anderson Fabry disease. Z Kardiol 2002;91:786-95. [Crossref] [PubMed]
- Putko BN, Wen K, Thompson RB, et al. Anderson-Fabry cardiomyopathy: prevalence, pathophysiology, diagnosis and treatment. Heart Fail Rev 2015;20:179-91. [Crossref] [PubMed]
- Beck M, Hughes D, Kampmann C, et al. Long-term effectiveness of agalsidase alfa enzyme replacement in Fabry disease: A Fabry Outcome Survey analysis. Mol Genet Metab Rep 2015;3:21-7. [Crossref] [PubMed]
- Kampmann C, Perrin A, Beck M. Effectiveness of agalsidase alfa enzyme replacement in Fabry disease: cardiac outcomes after 10 years' treatment. Orphanet J Rare Dis 2015;10:125. [Crossref] [PubMed]
- Cho A, Kim SJ, Lim BC, et al. Infantile Pompe disease: clinical and genetic characteristics with an experience of enzyme replacement therapy. J Child Neurol 2012;27:319-24. [Crossref] [PubMed]
- Chinnery P, Majamaa K, Turnbull D, et al. Treatment for mitochondrial disorders. Cochrane Database Syst Rev 2006.CD004426. [PubMed]
- Elliott PM, Anastasakis A, Borger MA, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733-79. [Crossref] [PubMed]
- Maron BJ, Mathenge R, Casey SA, et al. Clinical profile of hypertrophic cardiomyopathy identified de novo in rural communities. J Am Coll Cardiol 1999;33:1590-5. [Crossref] [PubMed]
- Zou Y, Song L, Wang Z, et al. Prevalence of idiopathic hypertrophic cardiomyopathy in China: a population-based echocardiographic analysis of 8080 adults. Am J Med 2004;116:14-8. [Crossref] [PubMed]
- Hada Y, Sakamoto T, Amano K, et al. Prevalence of hypertrophic cardiomyopathy in a population of adult Japanese workers as detected by echocardiographic screening. Am J Cardiol 1987;59:183-4. [Crossref] [PubMed]
- Tanigawa G, Jarcho JA, Kass S, et al. A molecular basis for familial hypertrophic cardiomyopathy: an alpha/beta cardiac myosin heavy chain hybrid gene. Cell 1990;62:991-8. [Crossref] [PubMed]
- Morita H, Nagai R, Seidman JG, et al. Sarcomere gene mutations in hypertrophy and heart failure. J Cardiovasc Transl Res 2010;3:297-303. [Crossref] [PubMed]
- Millat G, Bouvagnet P, Chevalier P, et al. Prevalence and spectrum of mutations in a cohort of 192 unrelated patients with hypertrophic cardiomyopathy. Eur J Med Genet 2010;53:261-7. [Crossref] [PubMed]
- Erdmann J, Daehmlow S, Wischke S, et al. Mutation spectrum in a large cohort of unrelated consecutive patients with hypertrophic cardiomyopathy. Clin Genet 2003;64:339-49. [Crossref] [PubMed]
- Richard P, Charron P, Carrier L, et al. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation 2003;107:2227-32. [Crossref] [PubMed]
- Knöll R, Hoshijima M, Hoffman HM, et al. The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell 2002;111:943-55. [Crossref] [PubMed]
- Walsh R, Buchan R, Wilk A, et al. Defining the genetic architecture of hypertrophic cardiomyopathy: re-evaluating the role of non-sarcomeric genes. Eur Heart J 2017;38:3461-8. [PubMed]
- Brito D, Richard P, Isnard R, et al. Familial hypertrophic cardiomyopathy: the same mutation, different prognosis. Comparison of two families with a long follow-up. Rev Port Cardiol 2003;22:1445-61. [PubMed]
- Mörner S, Richard P, Kazzam E, et al. Identification of the genotypes causing hypertrophic cardiomyopathy in northern Sweden. J Mol Cell Cardiol 2003;35:841-9. [Crossref] [PubMed]
- Marian AJ, Mares A Jr, Kelly DP, et al. Sudden cardiac death in hypertrophic cardiomyopathy. Variability in phenotypic expression of beta-myosin heavy chain mutations. Eur Heart J 1995;16:368-76. [Crossref] [PubMed]
- Lopes LR, Rahman MS, Elliott PM. A systematic review and meta-analysis of genotype-phenotype associations in patients with hypertrophic cardiomyopathy caused by sarcomeric protein mutations. Heart 2013;99:1800-11. [Crossref] [PubMed]
- Wolf CM, Moskowitz IP, Arno S, et al. Somatic events modify hypertrophic cardiomyopathy pathology and link hypertrophy to arrhythmia. Proc Natl Acad Sci U S A 2005;102:18123-8. [Crossref] [PubMed]
- Geisterfer-Lowrance AA, Christe M, Conner DA, et al. A mouse model of familial hypertrophic cardiomyopathy. Science 1996;272:731-4. [Crossref] [PubMed]
- Belus A, Piroddi N, Scellini B, et al. The familial hypertrophic cardiomyopathy-associated myosin mutation R403Q accelerates tension generation and relaxation of human cardiac myofibrils. J Physiol 2008;586:3639-44. [Crossref] [PubMed]
- Lan F, Lee AS, Liang P, et al. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell 2013;12:101-13. [Crossref] [PubMed]
- Palmer BM, Wang Y, Teekakirikul P, et al. Myofilament mechanical performance is enhanced by R403Q myosin in mouse myocardium independent of sex. Am J Physiol Heart Circ Physiol 2008;294:H1939-47. [Crossref] [PubMed]
- Green EM, Wakimoto H, Anderson RL, et al. A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science 2016;351:617-21. [Crossref] [PubMed]
- Tyska MJ, Hayes E, Giewat M, et al. Single-molecule mechanics of R403Q cardiac myosin isolated from the mouse model of familial hypertrophic cardiomyopathy. Circ Res 2000;86:737-44. [Crossref] [PubMed]
- Tardiff JC. Sarcomeric proteins and familial hypertrophic cardiomyopathy: linking mutations in structural proteins to complex cardiovascular phenotypes. Heart Fail Rev 2005;10:237-48. [Crossref] [PubMed]
- Sequeira V, Wijnker PJ, Nijenkamp LL, et al. Perturbed length-dependent activation in human hypertrophic cardiomyopathy with missense sarcomeric gene mutations. Circ Res 2013;112:1491-505. [Crossref] [PubMed]
- Linke WA. Sense and stretchability: the role of titin and titin-associated proteins in myocardial stress-sensing and mechanical dysfunction. Cardiovasc Res 2008;77:637-48. [PubMed]
- Fatkin D, McConnell BK, Mudd JO, et al. An abnormal Ca(2+) response in mutant sarcomere protein-mediated familial hypertrophic cardiomyopathy. J Clin Invest 2000;106:1351-9. [Crossref] [PubMed]
- Crilley JG, Boehm EA, Blair E, et al. Hypertrophic cardiomyopathy due to sarcomeric gene mutations is characterized by impaired energy metabolism irrespective of the degree of hypertrophy. J Am Coll Cardiol 2003;41:1776-82. [Crossref] [PubMed]
- Ashrafian H, McKenna WJ, Watkins H. Disease pathways and novel therapeutic targets in hypertrophic cardiomyopathy. Circ Res 2011;109:86-96. [Crossref] [PubMed]
- Ferrantini C, Belus A, Piroddi N, et al. Mechanical and energetic consequences of HCM-causing mutations. J Cardiovasc Transl Res 2009;2:441-51. [Crossref] [PubMed]
- Marston SB. How do mutations in contractile proteins cause the primary familial cardiomyopathies? J Cardiovasc Transl Res 2011;4:245-55. [Crossref] [PubMed]
- Popp MW, Maquat LE. Leveraging Rules of Nonsense-Mediated mRNA Decay for Genome Engineering and Personalized Medicine. Cell 2016;165:1319-22. [Crossref] [PubMed]
- Siwaszek A, Ukleja M, Dziembowski A. Proteins involved in the degradation of cytoplasmic mRNA in the major eukaryotic model systems. RNA Biol 2014;11:1122-36. [Crossref] [PubMed]
- Li RK, Li G, Mickle DA, et al. Overexpression of transforming growth factor-beta1 and insulin-like growth factor-I in patients with idiopathic hypertrophic cardiomyopathy. Circulation 1997;96:874-81. [Crossref] [PubMed]
- Yang W, Li Y, He F, et al. Microarray profiling of long non-coding RNA (lncRNA) associated with hypertrophic cardiomyopathy. BMC Cardiovasc Disord 2015;15:62. [Crossref] [PubMed]
- Konno T, Chen D, Wang L, et al. Heterogeneous myocyte enhancer factor-2 (Mef2) activation in myocytes predicts focal scarring in hypertrophic cardiomyopathy. Proc Natl Acad Sci U S A 2010;107:18097-102. [Crossref] [PubMed]
- Rohini A, Agrawal N, Koyani CN, et al. Molecular targets and regulators of cardiac hypertrophy. Pharmacol Res 2010;61:269-80. [Crossref] [PubMed]
- Teekakirikul P, Eminaga S, Toka O, et al. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-beta. J Clin Invest 2010;120:3520-9. [Crossref] [PubMed]
- Daw EW, Chen SN, Czernuszewicz G, et al. Genome-wide mapping of modifier chromosomal loci for human hypertrophic cardiomyopathy. Hum Mol Genet 2007;16:2463-71. [Crossref] [PubMed]
- Ambros V. The functions of animal microRNAs. Nature 2004;431:350-5. [Crossref] [PubMed]
- Laitala-Leinonen T. Update on the development of microRNA and siRNA molecules as regulators of cell physiology. Recent Pat DNA Gene Seq 2010;4:113-21. [Crossref] [PubMed]
- Kuster DW, Mulders J, Ten Cate FJ, et al. MicroRNA transcriptome profiling in cardiac tissue of hypertrophic cardiomyopathy patients with MYBPC3 mutations. J Mol Cell Cardiol 2013;65:59-66. [Crossref] [PubMed]
- Watkins H, Ashrafian H, Redwood C. Inherited cardiomyopathies. N Engl J Med 2011;364:1643-56. [Crossref] [PubMed]
- Maron MS, Olivotto I, Maron BJ, et al. The case for myocardial ischemia in hypertrophic cardiomyopathy. J Am Coll Cardiol 2009;54:866-75. [Crossref] [PubMed]
- Baudenbacher F, Schober T, Pinto JR, et al. Myofilament Ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J Clin Invest 2008;118:3893-903. [PubMed]
- Yacoub MH, Olivotto I, Cecchi F. 'End-stage' hypertrophic cardiomyopathy: from mystery to model. Nat Clin Pract Cardiovasc Med 2007;4:232-3. [Crossref] [PubMed]
- Birks EJ, Tansley PD, Hardy J, et al. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med 2006;355:1873-84. [Crossref] [PubMed]
- Tsoutsman T, Lam L, Semsarian C. Genes, calcium and modifying factors in hypertrophic cardiomyopathy. Clin Exp Pharmacol Physiol 2006;33:139-45. [Crossref] [PubMed]
- Saeed M, Link MS, Mahapatra S, et al. Analysis of intracardiac electrograms showing monomorphic ventricular tachycardia in patients with implantable cardioverter-defibrillators. Am J Cardiol 2000;85:580-7. [Crossref] [PubMed]
- Wolf CM, Berul CI. Molecular mechanisms of inherited arrhythmias. Curr Genomics 2008;9:160-8. [Crossref] [PubMed]
- Elliott P, Spirito P. Prevention of hypertrophic cardiomyopathy-related deaths: theory and practice. Heart 2008;94:1269-75. [Crossref] [PubMed]
- Kawara T, Derksen R, de Groot JR, et al. Activation delay after premature stimulation in chronically diseased human myocardium relates to the architecture of interstitial fibrosis. Circulation 2001;104:3069-75. [Crossref] [PubMed]
- Saumarez RC, Camm AJ, Panagos A, et al. Ventricular fibrillation in hypertrophic cardiomyopathy is associated with increased fractionation of paced right ventricular electrograms. Circulation 1992;86:467-74. [Crossref] [PubMed]
- Wood MA, Ellenbogen KA. Initiation of spontaneous ventricular tachycardia: from spark to fire. J Cardiovasc Electrophysiol 2000;11:727-9. [Crossref] [PubMed]
- Olivotto I, Cecchi F, Poggesi C, et al. Patterns of disease progression in hypertrophic cardiomyopathy: an individualized approach to clinical staging. Circ Heart Fail 2012;5:535-46. [Crossref] [PubMed]
- Biagini E, Coccolo F, Ferlito M, et al. Dilated-hypokinetic evolution of hypertrophic cardiomyopathy: prevalence, incidence, risk factors, and prognostic implications in pediatric and adult patients. J Am Coll Cardiol 2005;46:1543-50. [Crossref] [PubMed]
- Charron P, Dubourg O, Desnos M, et al. Diagnostic value of electrocardiography and echocardiography for familial hypertrophic cardiomyopathy in a genotyped adult population. Circulation 1997;96:214-9. [Crossref] [PubMed]
- Ho CY, Lopez B, Coelho-Filho OR, et al. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med 2010;363:552-63. [Crossref] [PubMed]
- Knollmann BC, Kirchhof P, Sirenko SG, et al. Familial hypertrophic cardiomyopathy-linked mutant troponin T causes stress-induced ventricular tachycardia and Ca2+-dependent action potential remodeling. Circ Res 2003;92:428-36. [Crossref] [PubMed]
- Harris KM, Spirito P, Maron MS, et al. Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation 2006;114:216-25. [Crossref] [PubMed]
- Olivotto I, Maron BJ, Appelbaum E, et al. Spectrum and clinical significance of systolic function and myocardial fibrosis assessed by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. Am J Cardiol 2010;106:261-7. [Crossref] [PubMed]
- Melacini P, Basso C, Angelini A, et al. Clinicopathological profiles of progressive heart failure in hypertrophic cardiomyopathy. Eur Heart J 2010;31:2111-23. [Crossref] [PubMed]
- Nistri S, Olivotto I, Betocchi S, et al. Prognostic significance of left atrial size in patients with hypertrophic cardiomyopathy (from the Italian Registry for Hypertrophic Cardiomyopathy). Am J Cardiol 2006;98:960-5. [Crossref] [PubMed]
- Maron BJ, Spirito P. Implications of left ventricular remodeling in hypertrophic cardiomyopathy. Am J Cardiol 1998;81:1339-44. [PubMed]
- Olivotto I, Cecchi F, Casey SA, et al. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation 2001;104:2517-24. [Crossref] [PubMed]
- Maron MS, Finley JJ, Bos JM, et al. Prevalence, clinical significance, and natural history of left ventricular apical aneurysms in hypertrophic cardiomyopathy. Circulation 2008;118:1541-9. [Crossref] [PubMed]
- Kubo T, Kitaoka H, Okawa M, et al. Combined measurements of cardiac troponin I and brain natriuretic peptide are useful for predicting adverse outcomes in hypertrophic cardiomyopathy. Circ J 2011;75:919-26. [Crossref] [PubMed]
- Biagini E, Spirito P, Leone O, et al. Heart transplantation in hypertrophic cardiomyopathy. Am J Cardiol 2008;101:387-92. [Crossref] [PubMed]
- Maron BJ, Ommen SR, Semsarian C, et al. Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol 2014;64:83-99. [Crossref] [PubMed]
- Kubo T, Gimeno JR, Bahl A, et al. Prevalence, clinical significance, and genetic basis of hypertrophic cardiomyopathy with restrictive phenotype. J Am Coll Cardiol 2007;49:2419-26. [Crossref] [PubMed]
- Caleshu C, Sakhuja R, Nussbaum RL, et al. Furthering the link between the sarcomere and primary cardiomyopathies: restrictive cardiomyopathy associated with multiple mutations in genes previously associated with hypertrophic or dilated cardiomyopathy. Am J Med Genet A 2011;155A:2229-35. [Crossref] [PubMed]
- Colan SD, Lipshultz SE, Lowe AM, et al. Epidemiology and cause-specific outcome of hypertrophic cardiomyopathy in children: findings from the Pediatric Cardiomyopathy Registry. Circulation 2007;115:773-81. [Crossref] [PubMed]
- Maron BJ, Casey SA, Hauser RG, et al. Clinical course of hypertrophic cardiomyopathy with survival to advanced age. J Am Coll Cardiol 2003;42:882-8. [Crossref] [PubMed]
- Schulze-Bahr E, Klaassen S, Abdul-Khaliq H, et al. Dtsch Med Wochenschr 2015;140:1538. [Molecular diagnosis for cardiovascular diseases]. [Crossref] [PubMed]
- Priori SG, Blomstrom-Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793-867. [Crossref] [PubMed]
- Ackerman MJ, Priori SG, Willems S, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm 2011;8:1308-39. [Crossref] [PubMed]
- Marian AJ, Braunwald E. Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ Res 2017;121:749-70. [Crossref] [PubMed]
- Morales A, Cowan J, Dagua J, et al. Family history: an essential tool for cardiovascular genetic medicine. Congest Heart Fail 2008;14:37-45. [Crossref] [PubMed]
- Marian AJ. Challenges in the Diagnosis of Anderson-Fabry Disease: A Deceptively Simple and Yet Complicated Genetic Disease. J Am Coll Cardiol 2016;68:1051-3. [Crossref] [PubMed]
- Campbell RM, Berger S, Drezner J. Sudden cardiac arrest in children and young athletes: the importance of a detailed personal and family history in the pre-participation evaluation. Br J Sports Med 2009;43:336-41. [Crossref] [PubMed]
- Liberthson RR. Sudden death from cardiac causes in children and young adults. N Engl J Med 1996;334:1039-44. [Crossref] [PubMed]
- Ranthe MF, Winkel BG, Andersen EW, et al. Risk of cardiovascular disease in family members of young sudden cardiac death victims. Eur Heart J 2013;34:503-11. [Crossref] [PubMed]
- Vincent GM. Sudden cardiac arrest in the young due to inherited arrhythmias: the importance of family care. Pacing Clin Electrophysiol 2009;32 Suppl 2:S19-22. [Crossref] [PubMed]
- Abrams DJ. How to develop a clinic for sudden cardiac arrest survivors and families of non-survivors. Cardiol Young 2017;27:S3-S9. [Crossref] [PubMed]
- Greaves SC, Roche AH, Neutze JM, et al. Inheritance of hypertrophic cardiomyopathy: a cross sectional and M mode echocardiographic study of 50 families. Br Heart J 1987;58:259-66. [Crossref] [PubMed]
- Adabag AS, Maron BJ, Appelbaum E, et al. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol 2008;51:1369-74. [Crossref] [PubMed]
- O'Mahony C, Lambiase PD, Quarta G, et al. The long-term survival and the risks and benefits of implantable cardioverter defibrillators in patients with hypertrophic cardiomyopathy. Heart 2012;98:116-25. [Crossref] [PubMed]
- Lin G, Nishimura RA, Gersh BJ, et al. Device complications and inappropriate implantable cardioverter defibrillator shocks in patients with hypertrophic cardiomyopathy. Heart 2009;95:709-14. [Crossref] [PubMed]
- Siontis KC, Geske JB, Ong K, et al. Atrial fibrillation in hypertrophic cardiomyopathy: prevalence, clinical correlations, and mortality in a large high-risk population. J Am Heart Assoc 2014;3:e001002. [Crossref] [PubMed]
- Debonnaire P, Joyce E, Hiemstra Y, et al. Left Atrial Size and Function in Hypertrophic Cardiomyopathy Patients and Risk of New-Onset Atrial Fibrillation. Circ Arrhythm Electrophysiol 2017;10. [Crossref] [PubMed]
- Guttmann OP, Pavlou M, O'Mahony C, et al. Prediction of thrombo-embolic risk in patients with hypertrophic cardiomyopathy (HCM Risk-CVA). Eur J Heart Fail 2015;17:837-45. [Crossref] [PubMed]
- Monserrat L, Elliott PM, Gimeno JR, et al. Non-sustained ventricular tachycardia in hypertrophic cardiomyopathy: an independent marker of sudden death risk in young patients. J Am Coll Cardiol 2003;42:873-9. [Crossref] [PubMed]
- Bunch TJ, Chandrasekaran K, Ehrsam JE, et al. Prognostic significance of exercise induced arrhythmias and echocardiographic variables in hypertrophic cardiomyopathy. Am J Cardiol 2007;99:835-8. [Crossref] [PubMed]
- Sheikh N, Papadakis M, Ghani S, et al. Comparison of electrocardiographic criteria for the detection of cardiac abnormalities in elite black and white athletes. Circulation 2014;129:1637-49. [Crossref] [PubMed]
- Corrado D, Pelliccia A, Heidbuchel H, et al. Recommendations for interpretation of 12-lead electrocardiogram in the athlete. Eur Heart J 2010;31:243-59. [Crossref] [PubMed]
- Nagueh SF, Zoghbi WA. Role of Imaging in the Evaluation of Patients at Risk for Sudden Cardiac Death: Genotype-Phenotype Intersection. JACC Cardiovasc Imaging 2015;8:828-45. [Crossref] [PubMed]
- Jan MF, Tajik AJ. Modern Imaging Techniques in Cardiomyopathies. Circ Res 2017;121:874-91. [Crossref] [PubMed]
- Colan SD. Hypertrophic cardiomyopathy in childhood. Heart Fail Clin 2010;6:433-44. vii-iii. [Crossref] [PubMed]
- Maron MS, Maron BJ, Harrigan C, et al. Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance. J Am Coll Cardiol 2009;54:220-8. [Crossref] [PubMed]
- Arad M, Penas-Lado M, Monserrat L, et al. Gene mutations in apical hypertrophic cardiomyopathy. Circulation 2005;112:2805-11. [Crossref] [PubMed]
- Malik R, Maron MS, Rastegar H, et al. Hypertrophic cardiomyopathy with right ventricular outflow tract and left ventricular intracavitary obstruction. Echocardiography 2014;31:682-5. [Crossref] [PubMed]
- Grazioli G, Usin D, Trucco E, et al. Differentiating hypertrophic cardiomyopathy from athlete's heart: An electrocardiographic and echocardiographic approach. J Electrocardiol 2016;49:539-44. [Crossref] [PubMed]
- Luijkx T, Cramer MJ, Prakken NH, et al. Sport category is an important determinant of cardiac adaptation: an MRI study. Br J Sports Med 2012;46:1119-24. [Crossref] [PubMed]
- Maron BJ, Doerer JJ, Haas TS, et al. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980-2006. Circulation 2009;119:1085-92. [Crossref] [PubMed]
- Caselli S, Maron MS, Urbano-Moral JA, et al. Differentiating left ventricular hypertrophy in athletes from that in patients with hypertrophic cardiomyopathy. Am J Cardiol 2014;114:1383-9. [Crossref] [PubMed]
- Petersen SE, Selvanayagam JB, Francis JM, et al. Differentiation of athlete's heart from pathological forms of cardiac hypertrophy by means of geometric indices derived from cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2005;7:551-8. [Crossref] [PubMed]
- Nugent AW, Daubeney PE, Chondros P, et al. Clinical features and outcomes of childhood hypertrophic cardiomyopathy: results from a national population-based study. Circulation 2005;112:1332-8. [Crossref] [PubMed]
- Michels M, Olivotto I, Asselbergs FW, et al. Life-long tailoring of management for patients with hypertrophic cardiomyopathy: Awareness and decision-making in changing scenarios. Neth Heart J 2017;25:186-99. [Crossref] [PubMed]
- Maron MS, Olivotto I, Harrigan C, et al. Mitral valve abnormalities identified by cardiovascular magnetic resonance represent a primary phenotypic expression of hypertrophic cardiomyopathy. Circulation 2011;124:40-7. [Crossref] [PubMed]
- Stewart S, Mason DT, Braunwald E. Impaired rate of left ventricular filling in idiopathic hypertrophic subaortic stenosis and valvular aortic stenosis. Circulation 1968;37:8-14. [Crossref] [PubMed]
- Biagini E, Spirito P, Rocchi G, et al. Prognostic implications of the Doppler restrictive filling pattern in hypertrophic cardiomyopathy. Am J Cardiol 2009;104:1727-31. [Crossref] [PubMed]
- Severino S, Caso P, Galderisi M, et al. Use of pulsed Doppler tissue imaging to assess regional left ventricular diastolic dysfunction in hypertrophic cardiomyopathy. Am J Cardiol 1998;82:1394-8. [Crossref] [PubMed]
- Mavrogeni S, Markousis-Mavrogenis G, Markussis V, et al. The Emerging Role of Cardiovascular Magnetic Resonance Imaging in the Evaluation of Metabolic Cardiomyopathies. Horm Metab Res 2015;47:623-32. [Crossref] [PubMed]
- Rickers C, Wilke NM, Jerosch-Herold M, et al. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation 2005;112:855-61. [Crossref] [PubMed]
- Moon JC, Fisher NG, McKenna WJ, et al. Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non-diagnostic echocardiography. Heart 2004;90:645-9. [Crossref] [PubMed]
- Rowin EJ, Maron MS, Lesser JR, et al. CMR with late gadolinium enhancement in genotype positive-phenotype negative hypertrophic cardiomyopathy. JACC Cardiovasc Imaging 2012;5:119-22. [Crossref] [PubMed]
- Chan RH, Maron BJ, Olivotto I, et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014;130:484-95. [Crossref] [PubMed]
- Briasoulis A, Mallikethi-Reddy S, Palla M, et al. Myocardial fibrosis on cardiac magnetic resonance and cardiac outcomes in hypertrophic cardiomyopathy: a meta-analysis. Heart 2015;101:1406-11. [Crossref] [PubMed]
- Ho CY, Abbasi SA, Neilan TG, et al. T1 measurements identify extracellular volume expansion in hypertrophic cardiomyopathy sarcomere mutation carriers with and without left ventricular hypertrophy. Circ Cardiovasc Imaging 2013;6:415-22. [Crossref] [PubMed]
- Ferreira PF, Kilner PJ, McGill LA, et al. In vivo cardiovascular magnetic resonance diffusion tensor imaging shows evidence of abnormal myocardial laminar orientations and mobility in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 2014;16:87. [Crossref] [PubMed]
- McGill LA, Ismail TF, Nielles-Vallespin S, et al. Reproducibility of in-vivo diffusion tensor cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 2012;14:86. [Crossref] [PubMed]
- Haland TF, Almaas VM, Hasselberg NE, et al. Strain echocardiography is related to fibrosis and ventricular arrhythmias in hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging 2016;17:613-21. [Crossref] [PubMed]
- Ho CY, Carlsen C, Thune JJ, et al. Echocardiographic strain imaging to assess early and late consequences of sarcomere mutations in hypertrophic cardiomyopathy. Circ Cardiovasc Genet 2009;2:314-21. [Crossref] [PubMed]
- Nagueh SF, McFalls J, Meyer D, et al. Tissue Doppler imaging predicts the development of hypertrophic cardiomyopathy in subjects with subclinical disease. Circulation 2003;108:395-8. [Crossref] [PubMed]
- Kramer CM, Reichek N, Ferrari VA, et al. Regional heterogeneity of function in hypertrophic cardiomyopathy. Circulation 1994;90:186-94. [Crossref] [PubMed]
- Urbano-Moral JA, Rowin EJ, Maron MS, et al. Investigation of global and regional myocardial mechanics with 3-dimensional speckle tracking echocardiography and relations to hypertrophy and fibrosis in hypertrophic cardiomyopathy. Circ Cardiovasc Imaging 2014;7:11-9. [Crossref] [PubMed]
- Arad M, Maron BJ, Gorham JM, et al. Glycogen storage diseases presenting as hypertrophic cardiomyopathy. N Engl J Med 2005;352:362-72. [Crossref] [PubMed]
- Ho CY, Charron P, Richard P, et al. Genetic advances in sarcomeric cardiomyopathies: state of the art. Cardiovasc Res 2015;105:397-408. [Crossref] [PubMed]
- Bagnall RD, Ingles J, Semsarian C. Molecular diagnostics of cardiomyopathies: the future is here. Circ Cardiovasc Genet 2011;4:103-4. [Crossref] [PubMed]
- Charron P, Arad M, Arbustini E, et al. Genetic counselling and testing in cardiomyopathies: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2010;31:2715-26. [Crossref] [PubMed]
- Van Driest SL, Ackerman MJ, Ommen SR, et al. Prevalence and severity of "benign" mutations in the beta-myosin heavy chain, cardiac troponin T, and alpha-tropomyosin genes in hypertrophic cardiomyopathy. Circulation 2002;106:3085-90. [Crossref] [PubMed]
- Gruner C, Ivanov J, Care M, et al. Toronto hypertrophic cardiomyopathy genotype score for prediction of a positive genotype in hypertrophic cardiomyopathy. Circ Cardiovasc Genet 2013;6:19-26. [Crossref] [PubMed]
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405-24. [Crossref] [PubMed]
- Amendola LM, Jarvik GP, Leo MC, et al. Performance of ACMG-AMP Variant-Interpretation Guidelines among Nine Laboratories in the Clinical Sequencing Exploratory Research Consortium. Am J Hum Genet 2016;98:1067-76. [Crossref] [PubMed]
- 1000 Genomes Project Consortium, Auton A, Brooks LD, et al. A global reference for human genetic variation. Nature 2015;526:68-74.
- Mellor G, Laksman ZWM, Tadros R, et al. Genetic Testing in the Evaluation of Unexplained Cardiac Arrest: From the CASPER (Cardiac Arrest Survivors With Preserved Ejection Fraction Registry). Circ Cardiovasc Genet 2017;10. [Crossref] [PubMed]
- Walsh R, Thomson KL, Ware JS, et al. Reassessment of Mendelian gene pathogenicity using 7,855 cardiomyopathy cases and 60,706 reference samples. Genet Med 2017;19:192-203. [Crossref] [PubMed]
- Lamke GT, Allen RD, Edwards WD, et al. Surgical pathology of subaortic septal myectomy associated with hypertrophic cardiomyopathy. A study of 204 cases (1996-2000). Cardiovasc Pathol 2003;12:149-58. [Crossref] [PubMed]
- Teare D. Asymmetrical hypertrophy of the heart in young adults. Br Heart J 1958;20:1-8. [Crossref] [PubMed]
- Davies MJ, McKenna WJ. Hypertrophic cardiomyopathy--pathology and pathogenesis. Histopathology 1995;26:493-500. [Crossref] [PubMed]
- Davies MJ. The current status of myocardial disarray in hypertrophic cardiomyopathy. Br Heart J 1984;51:361-3. [Crossref] [PubMed]
- van der Velden J, Ho CY, Tardiff JC, et al. Research priorities in sarcomeric cardiomyopathies. Cardiovasc Res 2015;105:449-56. [Crossref] [PubMed]
- Beale A, Macciocca I, Olaussen A, et al. Clinical benefits of a specialised clinic for hypertrophic cardiomyopathy. Intern Med J 2015;45:255-60. [Crossref] [PubMed]
- Day SM. Exercise in hypertrophic cardiomyopathy. J Cardiovasc Transl Res 2009;2:407-14. [Crossref] [PubMed]
- Ostman-Smith I, Wettrell G, Riesenfeld T. A cohort study of childhood hypertrophic cardiomyopathy: improved survival following high-dose beta-adrenoceptor antagonist treatment. J Am Coll Cardiol 1999;34:1813-22. [Crossref] [PubMed]
- Sherrid MV, Barac I, McKenna WJ, et al. Multicenter study of the efficacy and safety of disopyramide in obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2005;45:1251-8. [Crossref] [PubMed]
- Nishimura RA, Seggewiss H, Schaff HV. Hypertrophic Obstructive Cardiomyopathy: Surgical Myectomy and Septal Ablation. Circ Res 2017;121:771-83. [Crossref] [PubMed]
- Nishimura RA, Holmes DR Jr. Clinical practice. Hypertrophic obstructive cardiomyopathy. N Engl J Med 2004;350:1320-7. [Crossref] [PubMed]
- Lim DS, Lutucuta S, Bachireddy P, et al. Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation 2001;103:789-91. [Crossref] [PubMed]
- Shimada YJ, Passeri JJ, Baggish AL, et al. Effects of losartan on left ventricular hypertrophy and fibrosis in patients with nonobstructive hypertrophic cardiomyopathy. JACC Heart Fail 2013;1:480-7. [Crossref] [PubMed]
- Axelsson A, Iversen K, Vejlstrup N, et al. Functional effects of losartan in hypertrophic cardiomyopathy-a randomised clinical trial. Heart 2016;102:285-91. [Crossref] [PubMed]
- Ho CY, McMurray JJV, Cirino AL, et al. The Design of the Valsartan for Attenuating Disease Evolution in Early Sarcomeric Hypertrophic Cardiomyopathy (VANISH) Trial. Am Heart J 2017;187:145-55. [Crossref] [PubMed]
- Araujo AQ, Arteaga E, Ianni BM, et al. Effect of Losartan on left ventricular diastolic function in patients with nonobstructive hypertrophic cardiomyopathy. Am J Cardiol 2005;96:1563-7. [Crossref] [PubMed]
- Huang CY, Yang YH, Lin LY, et al. Renin-angiotensin-aldosterone blockade reduces atrial fibrillation in hypertrophic cardiomyopathy. Heart 2018;104:1276-83. [Crossref] [PubMed]
- Ho CY, Lakdawala NK, Cirino AL, et al. Diltiazem treatment for pre-clinical hypertrophic cardiomyopathy sarcomere mutation carriers: a pilot randomized trial to modify disease expression. JACC Heart Fail 2015;3:180-8. [Crossref] [PubMed]
- Westermann D, Knollmann BC, Steendijk P, et al. Diltiazem treatment prevents diastolic heart failure in mice with familial hypertrophic cardiomyopathy. Eur J Heart Fail 2006;8:115-21. [Crossref] [PubMed]
- Viola HM, Hool LC. The L-type Ca2+ channel: A mediator of hypertrophic cardiomyopathy. Channels (Austin) 2017;11:5-7. [Crossref] [PubMed]
- Mill JG, Milanez Mda C, de Resende MM, et al. Spironolactone prevents cardiac collagen proliferation after myocardial infarction in rats. Clin Exp Pharmacol Physiol 2003;30:739-44. [Crossref] [PubMed]
- Desai AS, Lewis EF, Li R, et al. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J 2011;162:966-972.e10. [Crossref] [PubMed]
- Marian AJ. Experimental therapies in hypertrophic cardiomyopathy. J Cardiovasc Transl Res 2009;2:483-92. [Crossref] [PubMed]
- Ashrafian H, Mason MJ, Mitchell AG. Regression of dilated-hypokinetic hypertrophic cardiomyopathy by biventricular cardiac pacing. Europace 2007;9:50-4. [Crossref] [PubMed]
- Maron BJ, Pelliccia A. The heart of trained athletes: cardiac remodeling and the risks of sports, including sudden death. Circulation 2006;114:1633-44. [Crossref] [PubMed]
- Knollmann BC, Blatt SA, Horton K, et al. Inotropic stimulation induces cardiac dysfunction in transgenic mice expressing a troponin T (I79N) mutation linked to familial hypertrophic cardiomyopathy. J Biol Chem 2001;276:10039-48. [Crossref] [PubMed]
- Maron BJ, Udelson JE, Bonow RO, et al. Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Task Force 3: Hypertrophic Cardiomyopathy, Arrhythmogenic Right Ventricular Cardiomyopathy and Other Cardiomyopathies, and Myocarditis: A Scientific Statement From the American Heart Association and American College of Cardiology. Circulation 2015;132:e273-80. [PubMed]
- Pelliccia A, Fagard R, Bjornstad HH, et al. Recommendations for competitive sports participation in athletes with cardiovascular disease: a consensus document from the Study Group of Sports Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J 2005;26:1422-45. [Crossref] [PubMed]
- Elliott PM, Poloniecki J, Dickie S, et al. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol 2000;36:2212-8. [Crossref] [PubMed]
- Spirito P, Autore C, Formisano F, et al. Risk of sudden death and outcome in patients with hypertrophic cardiomyopathy with benign presentation and without risk factors. Am J Cardiol 2014;113:1550-5. [Crossref] [PubMed]
- O'Mahony C, Tome-Esteban M, Lambiase PD, et al. A validation study of the 2003 American College of Cardiology/European Society of Cardiology and 2011 American College of Cardiology Foundation/American Heart Association risk stratification and treatment algorithms for sudden cardiac death in patients with hypertrophic cardiomyopathy. Heart 2013;99:534-41. [Crossref] [PubMed]
- Di Salvo G, Pacileo G, Limongelli G, et al. Non sustained ventricular tachycardia in hypertrophic cardiomyopathy and new ultrasonic derived parameters. J Am Soc Echocardiogr 2010;23:581-90. [Crossref] [PubMed]
- O'Mahony C, Lambiase PD, Rahman SM, et al. The relation of ventricular arrhythmia electrophysiological characteristics to cardiac phenotype and circadian patterns in hypertrophic cardiomyopathy. Europace 2012;14:724-33. [Crossref] [PubMed]
- Biagini E, Lorenzini M, Olivotto I, et al. Effects of myocardial fibrosis assessed by MRI on dynamic left ventricular outflow tract obstruction in patients with hypertrophic cardiomyopathy: a retrospective database analysis. BMJ Open 2012;2. [Crossref] [PubMed]
- Spirito P, Bellone P, Harris KM, et al. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med 2000;342:1778-85. [Crossref] [PubMed]
- Elliott PM, Gimeno Blanes JR, Mahon NG, et al. Relation between severity of left-ventricular hypertrophy and prognosis in patients with hypertrophic cardiomyopathy. Lancet 2001;357:420-4. [Crossref] [PubMed]
- Spirito P, Autore C, Rapezzi C, et al. Syncope and risk of sudden death in hypertrophic cardiomyopathy. Circulation 2009;119:1703-10. [Crossref] [PubMed]
- Gimeno JR, Tome-Esteban M, Lofiego C, et al. Exercise-induced ventricular arrhythmias and risk of sudden cardiac death in patients with hypertrophic cardiomyopathy. Eur Heart J 2009;30:2599-605. [Crossref] [PubMed]
- Maki S, Ikeda H, Muro A, et al. Predictors of sudden cardiac death in hypertrophic cardiomyopathy. Am J Cardiol 1998;82:774-8. [Crossref] [PubMed]
- D'Andrea A, Caso P, Severino S, et al. Prognostic value of intra-left ventricular electromechanical asynchrony in patients with hypertrophic cardiomyopathy. Eur Heart J 2006;27:1311-8. [Crossref] [PubMed]
- Oliva-Sandoval MJ, Ruiz-Espejo F, Monserrat L, et al. Insights into genotype-phenotype correlation in hypertrophic cardiomyopathy. Findings from 18 Spanish families with a single mutation in MYBPC3. Heart 2010;96:1980-4. [Crossref] [PubMed]
- Efthimiadis GK, Parcharidou DG, Giannakoulas G, et al. Left ventricular outflow tract obstruction as a risk factor for sudden cardiac death in hypertrophic cardiomyopathy. Am J Cardiol 2009;104:695-9. [Crossref] [PubMed]
- Elliott P, Gimeno J, Tome M, et al. Left ventricular outflow tract obstruction and sudden death in hypertrophic cardiomyopathy. Eur Heart J 2006;27:3073-author reply 3073-4. [Crossref] [PubMed]
- Maron MS, Olivotto I, Betocchi S, et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med 2003;348:295-303. [Crossref] [PubMed]
- O'Mahony C, Jichi F, Pavlou M, et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur Heart J 2014;35:2010-20. [Crossref] [PubMed]
- Ingles J, Doolan A, Chiu C, et al. Compound and double mutations in patients with hypertrophic cardiomyopathy: implications for genetic testing and counselling. J Med Genet 2005;42:e59. [Crossref] [PubMed]
- Moolman JC, Corfield VA, Posen B, et al. Sudden death due to troponin T mutations. J Am Coll Cardiol 1997;29:549-55. [Crossref] [PubMed]
- Maass AH, Ikeda K, Oberdorf-Maass S, et al. Hypertrophy, fibrosis, and sudden cardiac death in response to pathological stimuli in mice with mutations in cardiac troponin T. Circulation 2004;110:2102-9. [Crossref] [PubMed]
- Watkins H, McKenna WJ, Thierfelder L, et al. Mutations in the genes for cardiac troponin T and alpha-tropomyosin in hypertrophic cardiomyopathy. N Engl J Med 1995;332:1058-64. [Crossref] [PubMed]
- Hershberger RE, Lindenfeld J, Mestroni L, et al. Genetic evaluation of cardiomyopathy--a Heart Failure Society of America practice guideline. J Card Fail 2009;15:83-97. [Crossref] [PubMed]
- Nistri S, Olivotto I, Maron MS, et al. beta Blockers for prevention of exercise-induced left ventricular outflow tract obstruction in patients with hypertrophic cardiomyopathy. Am J Cardiol 2012;110:715-9. [Crossref] [PubMed]
- Gilligan DM, Chan WL, Joshi J, et al. A double-blind, placebo-controlled crossover trial of nadolol and verapamil in mild and moderately symptomatic hypertrophic cardiomyopathy. J Am Coll Cardiol 1993;21:1672-9. [Crossref] [PubMed]
- Senthil V, Chen SN, Tsybouleva N, et al. Prevention of cardiac hypertrophy by atorvastatin in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circ Res 2005;97:285-92. [Crossref] [PubMed]
- Patel R, Nagueh SF, Tsybouleva N, et al. Simvastatin induces regression of cardiac hypertrophy and fibrosis and improves cardiac function in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circulation 2001;104:317-24. [Crossref] [PubMed]
- Tsybouleva N, Zhang L, Chen S, et al. Aldosterone, through novel signaling proteins, is a fundamental molecular bridge between the genetic defect and the cardiac phenotype of hypertrophic cardiomyopathy. Circulation 2004;109:1284-91. [Crossref] [PubMed]
- Marian AJ, Senthil V, Chen SN, et al. Anti-fibrotic effects of antioxidant N-acetylcysteine in a mouse model of human hypertrophic cardiomyopathy mutation. J Am Coll Cardiol 2006;47:827-34. [Crossref] [PubMed]
- Nagueh SF, Lombardi R, Tan Y, et al. Atorvastatin and cardiac hypertrophy and function in hypertrophic cardiomyopathy: a pilot study. Eur J Clin Invest 2010;40:976-83. [Crossref] [PubMed]
- Marian AJ, Tan Y, Li L, et al. Hypertrophy Regression With N-Acetylcysteine in Hypertrophic Cardiomyopathy (HALT-HCM): A Randomized, Placebo-Controlled, Double-Blind Pilot Study. Circ Res 2018;122:1109-18. [Crossref] [PubMed]
- Jiang J, Wakimoto H, Seidman JG, et al. Allele-specific silencing of mutant Myh6 transcripts in mice suppresses hypertrophic cardiomyopathy. Science 2013;342:111-4. [Crossref] [PubMed]
- Thum T, Gross C, Fiedler J, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008;456:980-4. [Crossref] [PubMed]
- Roma-Rodrigues C, Raposo LR, Fernandes AR. MicroRNAs Based Therapy of Hypertrophic Cardiomyopathy: The Road Traveled So Far. Biomed Res Int 2015;2015:983290. [Crossref] [PubMed]
- Maron BJ, Rowin EJ, Udelson JE, et al. Clinical Spectrum and Management of Heart Failure in Hypertrophic Cardiomyopathy. JACC Heart Fail 2018;6:353-63. [Crossref] [PubMed]
- Sedaghat-Hamedani F, Kayvanpour E, Tugrul OF, et al. Clinical outcomes associated with sarcomere mutations in hypertrophic cardiomyopathy: a meta-analysis on 7675 individuals. Clin Res Cardiol 2018;107:30-41. [Crossref] [PubMed]
- Zuñiga Cisneros J, Stehlik J, Selzman CH, et al. Outcomes in Patients With Hypertrophic Cardiomyopathy Awaiting Heart Transplantation. Circ Heart Fail 2018;11:e004378. [Crossref] [PubMed]
- Morrow AG, Brockenbrough EC. Surgical treatment of idiopathic hypertrophic subaortic stenosis: technic and hemodynamic results of subaortic ventriculomyotomy. Ann Surg 1961;154:181-9. [Crossref] [PubMed]
- Redwood DR, Goldstein RE, Hirshfeld J, et al. Exercise performance after septal myotomy and myectomy in patients with obstructive hypertrophic cardiomyopathy. Am J Cardiol 1979;44:215-20. [Crossref] [PubMed]
- Kotkar KD, Said SM, Dearani JA, et al. Hypertrophic obstructive cardiomyopathy: the Mayo Clinic experience. Ann Cardiothorac Surg 2017;6:329-36. [Crossref] [PubMed]
- Schaff HV, Brown ML, Dearani JA, et al. Apical myectomy: a new surgical technique for management of severely symptomatic patients with apical hypertrophic cardiomyopathy. J Thorac Cardiovasc Surg 2010;139:634-40. [Crossref] [PubMed]
- Patel P, Dhillon A, Popovic ZB, et al. Left Ventricular Outflow Tract Obstruction in Hypertrophic Cardiomyopathy Patients Without Severe Septal Hypertrophy: Implications of Mitral Valve and Papillary Muscle Abnormalities Assessed Using Cardiac Magnetic Resonance and Echocardiography. Circ Cardiovasc Imaging 2015;8:e003132. [Crossref] [PubMed]
- Song HK, Turner J, Macfie R, et al. Routine Papillary Muscle Realignment and Septal Myectomy for Obstructive Hypertrophic Cardiomyopathy. Ann Thorac Surg 2018;106:670-5. [Crossref] [PubMed]
- Hong JH, Schaff HV, Nishimura RA, et al. Mitral Regurgitation in Patients With Hypertrophic Obstructive Cardiomyopathy: Implications for Concomitant Valve Procedures. J Am Coll Cardiol 2016;68:1497-504. [Crossref] [PubMed]
- Afanasyev A, Bogachev-Prokophiev A, Lenko E, et al. Myectomy with mitral valve repair versus replacement in adult patients with hypertrophic obstructive cardiomyopathy: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg 2019;28:465-72. [PubMed]
- Groben LP, Rosen E, Sanghvi K. Transradial alcohol septal ablation. J Invasive Cardiol 2014;26:E37-9. [PubMed]
- Isawa T, Tada N, Ootomo T, et al. Transradial Approach of Alcohol Septal Ablation Using a Sheathless Guiding Catheter: A Feasibility Study. J Invasive Cardiol 2015;27:E242-7. [PubMed]
- Faber L, Meissner A, Ziemssen P, et al. Percutaneous transluminal septal myocardial ablation for hypertrophic obstructive cardiomyopathy: long term follow up of the first series of 25 patients. Heart 2000;83:326-31. [Crossref] [PubMed]
- Ommen SR, Maron BJ, Olivotto I, et al. Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2005;46:470-6. [Crossref] [PubMed]
- Yang YJ, Fan CM, Yuan JQ, et al. Effectiveness of Alcohol Septal Ablation Versus Transaortic Extended Myectomy in Hypertrophic Cardiomyopathy with Midventricular Obstruction. J Interv Cardiol 2016;29:619-27. [Crossref] [PubMed]
- Maron BJ. Commentary and re-appraisal: surgical septal myectomy vs. alcohol ablation: after a decade of controversy and mismatch between clinical practice and guidelines. Prog Cardiovasc Dis 2012;54:523-8. [Crossref] [PubMed]
- Lombardi R, Betocchi S, Losi MA, et al. Myocardial collagen turnover in hypertrophic cardiomyopathy. Circulation 2003;108:1455-60. [Crossref] [PubMed]
- Kuusisto J, Karja V, Sipola P, et al. Low-grade inflammation and the phenotypic expression of myocardial fibrosis in hypertrophic cardiomyopathy. Heart 2012;98:1007-13. [Crossref] [PubMed]
- Moreno V, Hernandez-Romero D, Vilchez JA, et al. Serum levels of high-sensitivity troponin T: a novel marker for cardiac remodeling in hypertrophic cardiomyopathy. J Card Fail 2010;16:950-6. [Crossref] [PubMed]
- Roncarati R, Viviani Anselmi C, Losi MA, et al. Circulating miR-29a, among other up-regulated microRNAs, is the only biomarker for both hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2014;63:920-7. [Crossref] [PubMed]
- Derda AA, Thum S, Lorenzen JM, et al. Blood-based microRNA signatures differentiate various forms of cardiac hypertrophy. Int J Cardiol 2015;196:115-22. [Crossref] [PubMed]
- Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res 2012;110:483-95. [Crossref] [PubMed]


