
Relationship between monocyte to HDL cholesterol ratio and concomitant cardiovascular disease in Chinese Han patients with obstructive sleep apnea
Introduction
Obstructive sleep apnea (OSA) is a common condition characterized by recurrent partial or total obstructions of the upper airway, resulting in hypopnea or apnea (1,2). Several studies have revealed that OSA is an independent risk factor for cardiovascular diseases (CVD) (3-5). Chronic airway collapse leads to repeated hypoxia, which further contributes to increased sympathetic activation, oxidative stress, systemic inflammation, and endothelial dysfunction (2,6). Systemic inflammation with subsequent vascular damage has been implicated as the potential mechanism leading to the development of CVD morbidity in OSA (2,7,8).
During the past decade, blood cell count and its subsets have been reported to be consistently associated with the risk of CVD (9,10). It has been proved that monocytes and differentiated macrophages could modulate inflammatory cytokines and vessel remodeling in the process of CVD (11). High-density lipoprotein cholesterol (HDL-C) could suppress the monocyte activation, inhibit the proliferation and differentiation of monocyte progenitor cells, and mediate cholesterol efflux from macrophages (12,13). The monocyte to HDL-C ratio (MHR) has been addressed as a novel predictor and prognostic biomarker for CVD (11,12,14). Recent study found significant increase of total monocyte in bone marrow and peripheral blood upon OSA related chronic intermittent hypoxia (CIH) exposure (15), and OSA severity was independently associated with low HDL-C values as well (16). Atan et al. (17) reported MHR increased as OSA severity increased, which can be used as a new predictor for OSA. However, the association between MHR and CVD occurrence in patients with OSA still lacks clinical evidence and has not been well understood, especially in Chinese Han population. Thus, we conducted the present study to assess the correlation between MHR values and CVD occurrence in Chinese Han patients with OSA, and to further investigate the relevance of MHR as a marker to predict CVD occurrence in OSA patients.
Methods
Study population
We observed consecutive subjects who recorded polysomnography (PSG) at the Sleep Disorders Center of Ruijin Hospital affiliated to Shanghai JiaoTong University from September 2009 to January 2017. All subjects were adults of Chinese Han nationality. Demographic characteristics, smoking status, and previous history of chronic diseases were recorded. Body mass index (BMI) was calculated. Established CVD was defined as a history of coronary artery disease (i.e., previous myocardial infarction ≥90 days prior; stable or unstable angina with a diagnostic coronary angiography or positive exercise stress test; multivessel percutaneous angioplasty and/or stent ≥90 days prior; and/or multivessel coronary artery bypass graft ≥1 year prior) or a history of cerebrovascular disease (i.e., previous stroke ≥90 days prior or neurologist-diagnosed transient ischemic attack (TIA) of the brain or retina 30 days to 1 year prior). Exclusive criteria included patients with neural-muscular disease, sleep disorders other than OSA (e.g., central sleep apnea syndrome, restless leg syndrome, narcolepsy), previous treatment for OSA [e.g., continuous positive airway pressure (CPAP), surgery, and oral device, etc.], hypoxemic lung disease, stroke due to subarachnoid haemorrhage, hematologic disease, liver or kidney disease, chronic alcoholism, malignancy, pregnancy, acute and/or chronic infection, autoimmune disease, and anti-inflammatory medication use (such as corticosteroids, non-steroid anti-inflammatory drugs and immunosuppressive agents, etc.). In total, 657 subjects aged ≥18 years were included. The study was approved by the local ethics committee of Ruijin Hospital, and all the participants were provided with written informed consent.
Polysomnographic evaluation
All of the study participants performed polysomnography (PSG) (Alice 5, Philips Respironics, USA) in our sleep laboratory. PSG channels included four electroencephalogram (EEG) channels, submental electromyogram (EMG), two electrooculogram (EOG; right and left) channels, two electrocardiography (ECG) channels, pulse oxygen saturation, oral and nasal airflow, nasal air pressure, thoracic-abdominal respiratory movement, snoring microphone, and body position. According to the apnea-hypopnea index (AHI), subjects were categorized into four groups, including the control group (AHI <5), mild (AHI: 5–14.9), moderate (AHI: 15–29.9), and severe OSA (AHI ≥30) group (18). Hypopnea was defined as the peak signal excursions drop by ≥30% of pre-event baseline using nasal pressure for at least 10 seconds with a ≥4% oxygen desaturation from pre-event baseline. The percentage of sleep duration with SpO2 <90% (TS90), lowest pulse oxygen saturation (LSpO2), and mean oxygen saturation (mean SpO2) were also included.
Laboratory measurements
Fasting blood samples were taken in the morning after PSG monitoring. Blood samples were analyzed for complete blood count (CBC) using an automated hematology analysis device (CELL-DYN Ruby, Abbott Laboratories, USA). Serum fasting glucose and serum lipid profiles included total cholesterol (TC), triglycerides (TG), HDL-C and low-density lipoprotein cholesterol (LDL-C) were determined with AU5811 automatic biochemical analyzer (Beckman Coulter, USA). MHR = the monocyte count (103/μL)/HDL level (mg/dL) (11,12,19).
Statistical analysis
Data analysis was performed using statistical software (IBM SPSS Statistics for Windows, Version 22.0, USA). Continuous variables were reported as means ± standard deviations (SD), or median with inter-quartile range if variables were not normally distributed. Categorical variables were reported as constituent ratio. The significance of the mean differences between groups was assessed by Student t-test and one-way analysis of variance (ANOVA). For measurement data that were heteroscedastic or not normally distributed, Mann-Whitney U test and Kruskall-Wallis H test were performed for the comparison among groups. Constituent ratio among groups was compared by a chi square test. The relationships were determined using Pearson’s rank correlation. The effect of various variables on CVD was analyzed with logistic regression analysis. Receiver-operating characteristic (ROC) curve was used to estimate the predictive validity, and to determine the optimal MHR cut-off value (20). P value<0.05 was considered statistically significant.
Results
Demographic characteristics
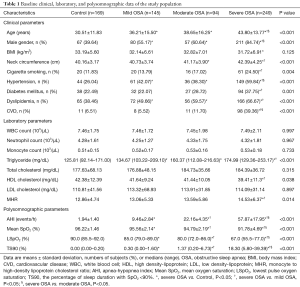
A total of 657 individuals (415 males, aged 37.97±15.02 years) were enrolled in the study, with average BMI 32.35±6.56 kg/m2. All clinical variables collected were summarized in Table 1. The occurrence of CVD increased along with the severity of OSA (mild 5.52%, moderate 11.70%, and severe 39.36%, P<0.001; Table 1). Compared with the control group, the odds ratio (OR) of CVD was 4.53 (95% CI: 2.38–8.64; P<0.001) in the OSA group, and 9.32 in the severe OSA group (95% CI: 4.81–18.07; P<0.001).
Full table
Correlations between PSG parameters and MHR
As shown in Table 1, monocyte counts and MHR values were found elevated in parallel with the increase of OSA severity. Serum HDL-C levels in severe OSA group were the lowest among all 4 groups (P<0.05; Table 1). The MHR levels in severe OSA group were higher than those in the control group and mild group (P=0.002 and P=0.020, respectively). Moreover, MHR was positively correlated with AHI (r=0.167, P<0.001) and TS90 (r=0.110, P=0.005), while negatively with LSpO2 (r=−0.123, P=0.002) and mean SpO2 (r=−0.134, P=0.001).
The association between MHR and CVD in OSA subjects
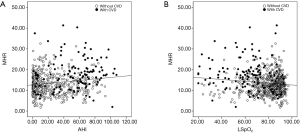
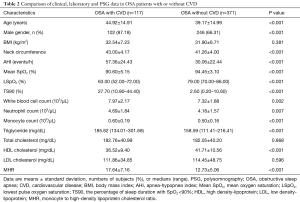
In addition, the MHR values in OSA subjects were positively correlated with CVD occurrence (r=0.349, P<0.001). The levels of MHR were mostly higher in CVD patients with similar severity of AHI and LSpO2 (Figure 1). We further compared the differences between OSA patients with (n=117) and without CVD (n=371) in Table 2. OSA patients with CVD were found to have higher AHI and lower LSpO2, while the MHR values of OSA patients with CVD were significantly higher than those without CVD (17.64±7.16 vs. 12.73±5.06, P<0.001; Table 2). For the CVD patients, the levels of MHR in severe OSA group were found the highest among all OSA groups (mild 12.40±4.59, moderate 17.26±8.48, and severe 18.11±7.06, respectively).
Full table
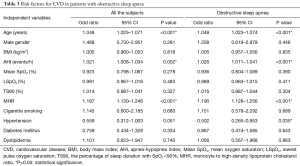
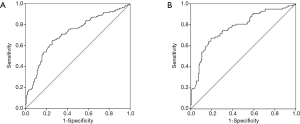
In logistic regression analysis for the risk factors of CVD in OSA patients, MHR was determined as an independent predictor of CVD (OR =1.190, 95% CI: 1.128–1.256, P<0.001; Table 3). For the prediction of CVD in OSA patients, the ROC curve analysis performed the cut-off value of MHR (>15.364) with the greatest sum of sensitivity (0.65) and specificity (0.744), and area under the curve value (AUC) of 0.720 (95% CI: 0.664–0.775, P<0.001; Figure 2A). In addition, the AUC for MHR to identify CVD in severe OSA patients was 0.774 (95% CI: 0.712–0.835, P<0.001); the cut-off value was 15.362, with the greatest sum of sensitivity (0.673) and specificity (0.801) (Figure 2B).
Full table
Discussion
The present study mainly examined the relationship between MHR and CVD risk in Chinese Han patients with OSA. To the best of our knowledge, this is the first study to investigate this association among Chinese populations. The results showed that MHR increased along with the severity of OSA, and the MHR values were significantly higher in CVD patients with OSA, especially in those with severe OSA. Notably, we found that MHR act as an independent predictor of CVD in Chinese Han patients with OSA after adjusting for known important confounding variables including age, sex, BMI, smoking, hypertension, etc.
OSA is an independent risk factor for cardiovascular events (3-5). Chronic intermittent hypoxia (CIH) is a hallmark of OSA, which induces a state of low-grade circulation and systemic inflammation in OSA patients (2), leading to the cardiovascular damage (7,8,21-23). In our study, the risk of CVD in OSA patients increased more than four-fold compared with the control group. In addition, the risk of exhibiting CVD was 9.32-fold higher in severe OSA group, which may be related to the aggravated systemic inflammation resulted from OSA.
Monocytes and differentiated macrophages are essential components of innate immunity and can modulate the secretion of inflammatory cytokines and tissue remodeling, resulting in chronic inflammation and cardiovascular events (21,23,24). The effect of HDL-C on monocytes has also been proposed. HDL-C inhibits monocyte activation and extravagation by down-regulating the expression of CD11b (25), which counter acts the migration of macrophages and promotes efflux of oxidized cholesterol from these cells (11,23). HDL-C has been proven to have anti-inflammatory, antioxidant, and antithrombotic effects (26). Therefore, MHR may represent the balance between inflammatory and anti-inflammatory factors (25). Several studies suggested that MHR is related to cardiovascular outcomes, and is an independent predictor of stent thrombosis in acute ST-segment elevation myocardial infarction (STEMI) patients (27). Increased MHR is also in accordance with the higher recurrence of atrial fibrillation (28), existence of slow coronary flow (29), thrombolysis in myocardial infarction (TIMI) score (23), and in-hospital major adverse cardiovascular events (MACEs) in patients with STEMI (30).
Recently, Alvarez-Martins et al. found an increase in the CD11b+ myeloid cells, the majority of which are monocytes, in CIH exposed rats (15). The European Sleep Apnea Database (ESADA) cohort, included 8592 patients, identified a strong association between lower HDL-C and OSA severity (16). We attempted to determine the role of MHR in CVD occurrence of patients with OSA. In our study, significantly higher MHR values were observed in patients with OSA when compared with the control group. The MHR values had a linear correlation with AHI, and the severity of hypoxemia defined by mean SpO2, LSpO2 and TS90, which was consistent with previous studies (17,19). Furthermore, the levels of MHR were significantly higher in OSA patients with CVD, especially in severe OSA group. Logistic regression analysis demonstrated that MHR was independently associated with the incidence of CVD, which indicated that an increased MHR might be a predictor for the development and progression of cardiovascular events in OSA patients. The finding was consistent with Kanbay’s report, which reckoned MHR as an independent predictor of major cardiovascular injuries in chronic kidney disease patients (31). As for the cut-off value of MHR in predicting CVDs, different values varying from 14.73 to 21.3 have been reported (19,24). Geovanini et al. pointed out the pathways linking OSA to CVD are complex and the associations between them vary across population groups (32). We herein found that MHR of 15.36 could predict CVD occurrence in Chinese Han patients with OSA, with the greatest sum of sensitivity (0.65) and specificity (0.744). Moreover, with the increasing severity of OSA, the cutoff value of MHR for detecting CVD in severe OSA patients with a sensitivity of 0.673 and a specificity of 0.801, which suggested that the predictive value of MHR may be more remarkable in CVD with severe OSA patients.
Several limitations should be mentioned. First, given the cross-sectional nature of this single center study, inferences on the cause-and-effect sequence between OSA and CVD are not possible. Second, we did not study MHR changes before and after CPAP treatment. Thus, further well-designed, multi-center, prospective interventional clinical trials with CPAP treatment are needed in the future.
Conclusions
In summary, the results of our study implied the importance of MHR in the prediction of CVD among Chinese Han patients with OSA from a relatively large population. It is suggested that MHR, which is an easy and available test, might be an available biomarker to evaluate CVD risk in OSA patients, especially in severe OSA patients.
Acknowledgments
Funding: This study was supported by National Nature Science Foundation of China Grants (81770084and 81570082), National Key Technology Research and Development Program of China (2018YFC1311900) and Shanghai Key Discipline for Respiratory Disease (2017ZZ02014).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All the authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study has been approved by the Ethics Committee of Ruijin Hospital affiliated to Shanghai JiaoTong University School of Medicine ([2018] No.107). All the subjects gave their informed consent prior to their inclusion in the study.
References
- Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 2015;3:310-8. [Crossref] [PubMed]
- Turnbull CD. Intermittent hypoxia, cardiovascular disease and obstructive sleep apnoea. J Thorac Dis 2018;10:S33-9. [Crossref] [PubMed]
- Gami AS, Olson EJ, Shen WK, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol 2013;62:610-6. [Crossref] [PubMed]
- Kendzerska T, Gershon AS, Hawker G, et al. Obstructive sleep apnea and risk of cardiovascular events and all-cause mortality: a decade-long historical cohort study. PLoS Med 2014;11:e1001599. [Crossref] [PubMed]
- McNicholas WT, Bonsigore MR. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J 2007;29:156-78. [Crossref] [PubMed]
- Bouloukaki I, Mermigkis C, Tzanakis N, et al. Evaluation of Inflammatory Markers in a Large Sample of Obstructive Sleep Apnea Patients without Comorbidities. Mediators Inflamm 2017;2017:4573756. [Crossref] [PubMed]
- Chami HA, Fontes JD, Vasan RS, et al. Vascular inflammation and sleep disordered breathing in a community-based cohort. Sleep 2013;36:763-8c. [Crossref] [PubMed]
- Kıvanc T, Kulaksizoglu S, Lakadamyali H, et al. Importance of laboratory parameters in patients with obstructive sleep apnea and their relationship with cardiovascular diseases. J Clin Lab Anal 2018;32. [Crossref] [PubMed]
- Ates AH, Canpolat U, Yorgun H, et al. Total white blood cell count is associated with the presence, severity and extent of coronary atherosclerosis detected by dual-source multislice computed tomographic coronary angiography. Cardiol J 2011;18:371-7. [PubMed]
- Kim J, Song TJ, Park JH, et al. Different prognostic value of white blood cell subtypes in patients with acute cerebral infarction. Atherosclerosis 2012;222:464-7. [Crossref] [PubMed]
- Zhang Y, Li S, Guo YL, et al. Is monocyte to HDL ratio superior to monocyte count in predicting the cardiovascular outcomes: evidence from a large cohort of Chinese patients undergoing coronary angiography. Ann Med 2016;48:305-12. [Crossref] [PubMed]
- Cetin MS, Ozcan Cetin EH, Kalender E, et al. Monocyte to HDL Cholesterol Ratio Predicts Coronary Artery Disease Severity and Future Major Cardiovascular Adverse Events in Acute Coronary Syndrome. Heart Lung Circ 2016;25:1077-86. [Crossref] [PubMed]
- Wang P, Wang Y, Ma W, et al. High-density lipoprotein cholesterol and intracoronary thrombosis burden. Coron Artery Dis 2013;24:1-5. [Crossref] [PubMed]
- Ganjali S, Gotto AM Jr, Ruscica M, et al. Monocyte-to-HDL-cholesterol ratio as a prognostic marker in cardiovascular diseases. J Cell Physiol 2018;233:9237-46. [Crossref] [PubMed]
- Alvarez-Martins I, Remedio L, Matias I, et al. The impact of chronic intermittent hypoxia on hematopoiesis and the bone marrow microenvironment. Pflugers Arch 2016;468:919-32. [Crossref] [PubMed]
- Gündüz C, Basoglu OK, Hedner J, et al. Obstructive sleep apnoea independently predicts lipid levels: Data from the European Sleep Apnea Database. Respirology 2018;23:1180-9. [Crossref] [PubMed]
- Atan D, Kundi FCS, Ozcan KM, et al. A New Predictor for Obstructive Sleep Apnea Syndrome: Monocyte to HDL Ratio. Indian J Otolaryngol Head Neck Surg 2017;69:142-6. [Crossref] [PubMed]
- Ruehland WR, Rochford PD, O'Donoghue FJ, et al. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep 2009;32:150-7. [Crossref] [PubMed]
- Inonu Koseoglu H, Pazarli AC, Kanbay A, et al. Monocyte Count/HDL Cholesterol Ratio and Cardiovascular Disease in Patients With Obstructive Sleep Apnea Syndrome: A Multicenter Study. Clin Appl Thromb Hemost 2018;24:139-44. [Crossref] [PubMed]
- Bantis LE, Nakas CT, Reiser B. Construction of confidence regions in the ROC space after the estimation of the optimal Youden index-based cut-off point. Biometrics 2014;70:212-23. [Crossref] [PubMed]
- Moro-García MA, Echeverria A, Galan-Artimez MC, et al. Immunosenescence and inflammation characterize chronic heart failure patients with more advanced disease. Int J Cardiol 2014;174:590-9. [Crossref] [PubMed]
- Padeletti M, Zaca V, Mondillo S, et al. Sleep-disordered breathing increases the risk of arrhythmias. J Cardiovasc Med (Hagerstown) 2014;15:411-6. [Crossref] [PubMed]
- Sercelik A, Besnili AF. Increased monocyte to high-density lipoprotein cholesterol ratio is associated with TIMI risk score in patients with ST-segment elevation myocardial infarction. Rev Port Cardiol 2018;37:217-23. [Crossref] [PubMed]
- Wei XB, Chen F, Huang JL, et al. Novel Risk Biomarker for Infective Endocarditis Patients With Normal Left Ventricular Ejection Fraction- Monocyte to High-Density Lipoprotein Cholesterol Ratio. Circ J 2017;82:283-8. [Crossref] [PubMed]
- Baek K, Chung I. Cadmium Exposure Is Associated with Monocyte Count and Monocyte to HDL Ratio, a Marker of Inflammation and Future Cardiovascular Disease in the Male Population. J Korean Med Sci 2017;32:1415-22. [Crossref] [PubMed]
- Barter PJ, Nicholls S, Rye KA, et al. Antiinflammatory properties of HDL. Circ Res 2004;95:764-72. [Crossref] [PubMed]
- Cetin EH, Cetin MS, Canpolat U, et al. Monocyte/HDL-cholesterol ratio predicts the definite stent thrombosis after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Biomark Med 2015;9:967-77. [Crossref] [PubMed]
- Canpolat U, Aytemir K, Yorgun H, et al. The role of preprocedural monocyte-to-high-density lipoprotein ratio in prediction of atrial fibrillation recurrence after cryoballoon-based catheter ablation. Europace 2015;17:1807-15. [Crossref] [PubMed]
- Canpolat U, Cetin EH, Cetin S, et al. Association of Monocyte-to-HDL Cholesterol Ratio with Slow Coronary Flow is Linked to Systemic Inflammation. Clin Appl Thromb Hemost 2016;22:476-82. [Crossref] [PubMed]
- Karataş MB, Canga Y, Ozcan KS, et al. Monocyte to high-density lipoprotein ratio as a new prognostic marker in patients with STEMI undergoing primary percutaneous coronary intervention. Am J Emerg Med 2016;34:240-4. [Crossref] [PubMed]
- Kanbay M, Solak Y, Unal HU, et al. Monocyte count/HDL cholesterol ratio and cardiovascular events in patients with chronic kidney disease. Int Urol Nephrol 2014;46:1619-25. [Crossref] [PubMed]
- Geovanini GR, Wang R, Weng J, et al. Association between Obstructive Sleep Apnea and Cardiovascular Risk Factors: Variation by Age, Sex, and Race. The Multi-Ethnic Study of Atherosclerosis. Ann Am Thorac Soc 2018;15:970-7. [Crossref] [PubMed]


