
Potassium selenocyanoacetate reduces the blood triacylglycerol and atherosclerotic plaques in high-fat-dieted mice
Introduction
Overconsumption of a diet high in fat have plagued both developed and developing countries in recent years. High-fat diet can lead to obesity which increased the risk of several diseases, most notably cardiovascular disease, diabetes, and cancers (1). In the area of cardiovascular disease, it is generally known that high-fat diet-induced hyperlipidemia play a causal role in the development of atherosclerosis, which is the major cause of cardiovascular disease, and characterized by a chronic inflammatory condition with immune competent cells in lesions producing mainly pro-inflammatory cytokines (2). Current management of lipid levels in atherosclerosis is using lipid-lowering drug therapy, such as statins and fibrates and which have side effects on kidney and liver when used in long-term (3). Hence, it is necessity to explore efficient but low-side effect drug candidate for atherosclerosis. Some studies suggested vitamin E inhibits atherosclerosis by anti-oxidation (4). And it is generally accepted that selenium has anti-oxidation function. Thus, we speculated that potassium selencyanoacetate, a kind of organic selenium which is synthesized through structural modification and its synthetic products can offer excellent outcomes in disease prevention with minimal toxicity and few side effects, can be effectively on atherosclerosis.
In this study, we investigated the protective effect of potassium selencyanoacetate on the blood triacylglycerol (TG) and atherosclerotic plaques in high-fat-dieted mice.
Methods
Laboratory animals
A total of 40 healthy, clean and specific pathogen free (SPF)-grade ApoE−/− male mice, aged 8–10 weeks, weighing 22–33 g, were purchased from the Center of Animal Experiments, Wuhan University (Hubei, China). The mice were maintained in SPF-grade animal housing facility, with supplied of clean drinking water, and a 12-h light (7:00–19:00) and dark (19:00–7:00) cycle. All the mice fed adaptively for 1 week prior to the experiments. This animal experiment was approved by the Ethics Committee of Animal Protection and Animal Experiment of Wuhan University (Hubei, China).
Construction and grouping of animal models of atherosclerosis
After 1 week for adapted, forty mice were randomly divided into the treatment group, n=20) and the atherosclerosis group (control group, n=20). The control group was given a high-fat diet (recipe: 15.8% protein, 40% fat and 68.8% carbohydrate) and 1.5 mL normal saline, and the treatment group was given a high-fat diet and potassium selencyanoacetate at 4.63 mg/kg/day (5). After 16 weeks of feeding and 12 hours of fasting, animals in each group were euthanatized by anesthetic inhalation.
Serum extraction
Blood was collected from the orbit used with EP tube and stood at room temperature for 1.5 h making the blood coagulated naturally, and centrifuged at 4,000×g for 30 min at 4 °C to separate the serum and then a 40 mL of each of the three tubes of serum was transferred into a 0.2 mL of sterile EP tube, and the samples were labeled and stored at −80 °C.
Detection of blood lipids
Blood Lipids in the separated serum was tested by automatic biochemical analyzer, including total cholesterol (TC), TG, HDL cholesterol (HDL-Ch) and LDL cholesterol (LDL-Ch), alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (Cr), and plasma urea.
Aortic tree separation
The weight of mice was recorded before death. The aortic tree was collected from the beginning of the aortic arch to the left and right branches of the iliac artery, pured H2O for 10 min, treated with 60% isopropanol solution for 2 min, added to the oil red O staining solution sample for 1 h, and then washed with a 60% isopropanol solution three times, each wash for 1 min until the background staining was removed, removed residual adventitial fat under a dissecting microscope, placed on a black-wax anatomical plate for analysis of atherosclerotic plaque.
Atherosclerotic plaque analysis
Aortic tree was detected for forming of the atherosclerotic plaque. The results of atherosclerotic plaque formations and serological indexes were analyzed using image processing software Image J or Image-Pro Plus, and the data were plotted using the GraphPad Prism software.
Statistical analysis
Data were expressed as mean ± standard deviation (SD) and analyzed by SPSS 20.0 statistical software. The comparisons between the two groups were performed using Student’s t-test, and P<0.05 was considered as the statistical significance.
Results
Effects of potassium selencyanoacetate on the formation of atherosclerotic plaques in mice fed with a high-fat diet
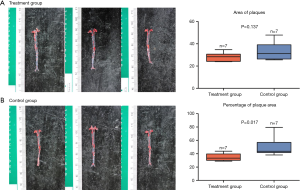
The red O oil staining showed no significant reduction in the area of atherosclerotic plaques in the treatment group as compared to the control group (28.5±3.8 vs. 33.7±7.8, P=0.137) (Figure 1A). However, the percentage of plaque area in the treatment group was significantly reduced (51.0±14.3 vs. 34.8±5.6, P=0.017) (Figure 1B) compared with that of the control group.
Effects of potassium selencyanoacetate on the TC, TG, HDL-Ch, and LDL-Ch levels in atherosclerotic mice
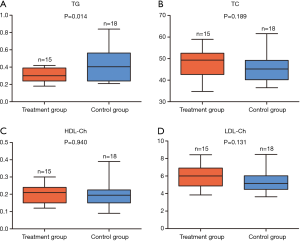
In order to explore the effects of potassium selencyanoacetate on the blood lipid profiles of atherosclerotic mice, we carried out assays to detect the levels of four blood lipids markers in the two groups of mice, including TC, TG, HDL-Ch and LDL-Ch.
As compared to the control group, the TG levels decreased significantly in the treatment group (0.296±0.081 vs. 0.435±0.193, P=0.014) (Figure 2A). However, the levels of TC (48.58±6.75 vs. 45.49±6.46, P=0.189) (Figure 2B), HDL-Ch (0.200±0.050 vs. 0.202±0.071, P=0.940) (Figure 2C), and LDL-Ch (5.948±1.269 vs. 5.265±1.254, P=0.131) (Figure 2D) did not exhibit any notable changes.
Effects of potassium selencyanoacetate on the levels of ALT, AST, and plasma urea in atherosclerotic mice
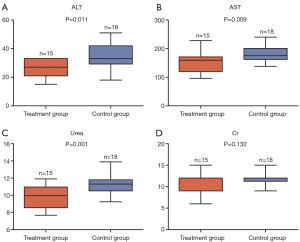
To explore the effects of potassium selencyanoacetate on the liver and kidney functions of atherosclerotic mice, we measured the levels of ALT, AST, Cr, and plasma urea in the two groups. Figure 3 shows that the levels of ALT (34.0±8.7 vs. 26.8±5.8, P=0.011), AST (150.8±33.6 vs. 180.0±26.6, P=0.009) and plasma urea (9.8±1.3 vs. 11.4±1.2, P=0.001) were significantly decreased in the treatment group as compared to those of the control group (Figure 3A,B,C). However, the Cr levels showed no significant difference between the two groups (10.4±2.5 vs. 11.5±1.5, P=0.132) (Figure 3D).
Discussion
In the current study, we found that during the feeding process of the animals with the high-fat diet plus potassium selencyanoacetate, potassium selencyanoacetate could reduce the level of TG in mice, indicating that potassium selencyanoacetate may slow down the development of atherosclerosis, which could be achieved through regulating the lipid TG level. Meanwhile, the levels of ALT, AST, and urea in the treated group showed the trends of reduction, indicating that the organic selenium can help the activities of the liver and kidney to remain normal.
High-fat diet is the most common phenomenon in humans in recent years. Furthermore, high-fat diet induced dyslipidemia, and then leads to a range of health-threatening diseases, especially atherosclerosis cardiovascular disease (ASCVD), which is the major causes of death and disability of cardiovascular disease. ASCVD is defined as nonfatal acute myocardial infarction or coronary disease death or fatal or nonfatal stroke (6). The primary goal is to using lipid-lowering drugs in treating ASCVD, such as statins and fibrates. As we known, statins and fibrates have side effects on kidney and liver when used in long-term (3). Therefore, new approaches are needed to provide with safe and reliable treatment. In recent years, some studies have shown that vitamin E inhibits atherosclerosis by anti-oxidation (4).
Selenium exists in nature with two ways, organic selenium and inorganic selenium. Selenium is one of the essential trace minerals, which participates in various metabolic processes in the human body. Selenium is incorporated into selenoproteins, which mainly includes glutathione peroxidases, thioredoxin reductase 1, selenoprotein P, selenoprotein S, plays a crucial role in preventing atherosclerosis (7). Some animals’ studies have confirmed its underlying mechanisms (7), including inhibiting oxidative stress, modulating inflammation, suppressing endothelial dysfunction, and protecting vascular cells against apoptosis and calcification.
Current studies of using organic selenium as a drug treating atherosclerosis are diphenyl diselenide (DD) and ebselen [2-phenyl-1, 2-benzisoselenazol-3 (2H)-One] (8,9). Ward et al. (8) found that the protective effects of DD on oxidized low-density lipoprotein (oxLDL)-induced endothelial dysfunction were achieved through the following three aspects: (I) DD at concentrations of 0.1–1 mM protected bovine endothelial aortic cells by inhibiting the oxLDL-induced production of active reactive oxygen species and reducing the formation of reduced glutathione, (II) DD (mM) maximally improved mitochondrial respiratory function and prevented mitochondrial damage from oxLDL, and (III) DD prevented oxLDL-induced cell apoptosis. In a study on ebselen, Ali et al. (9) pointed out that ebselen has both antioxidant and glutathione peroxidase activities, and it reduced the H2O2-induced apoptosis of human umbilical vein endothelial cells. Potassium selencyanoacetate (NCSeCH2COOK, C3H2KNO2Se) also one of organic selenium, synthesized through structural modification and its synthetic products can offer excellent outcomes in disease prevention with minimal toxicity and few side effects. Therefore, because of the anti-oxidation function of selenium, we speculated that potassium selencyanoacetate can be effectively on atherosclerosis. Further, to the best of our knowledge, it is the first study exploring the effects of potassium selencyanoacetate reduce the blood TG and atherosclerotic plaques in high-fat-dieted mice.
Only the level of TG, a major component of triglyceride lipoproteins, decreased in the mice treated with potassium selencyanoacetate. According to a recent study, the definitive correlation between the serum selenium and the blood lipid contents remains unclear. For instance, a cross-sectional data analysis of serum selenium levels, blood lipid concentrations and other related markers in 8,189 rural Chinese individuals has showed that elevated levels of serum selenium were not only associated with increased concentrations of TC, TG, HDL-Ch, and LDL-Ch but also related to an increased risk of high cholesterol and HDL dyslipidemia (10). However, a meta-analysis examining the association between selenium and lipid metabolism has showed that a selenium-associated improvement in the TC levels was statistically significant (11). Although it could not have robust evidence of the role of TG-lowering therapy in reducing the risk of ASCVD. The Japan Atherosclerotic Society guidelines for the prevention of ASCVD have established TG levels <150 mg/dL in patient with or without coronary artery disease (12). And future clinical trials are needed to determine whether serum TG-lowering therapy reduces the risk of ASCVD in patients with elevated serum TG levels (13). In addition, the data presented that the percentage of plaque area, representing levels of vascular stenosis, is statistically significant. Recently, an increasingly emerging evidence from many studies has suggested that hypertriglyceridemia is a positive causal factor in increasing the size of the atherosclerotic plaques (14-17), which was confirmed in both animal studies and epidemiological analyses. The existence of residual triglyceride-containing lipoproteins in human atherosclerotic plaques were discovered (18). Moreover, reducing triglyceride levels can slow down the progression of atherosclerosis, the molecular mechanisms underlying this process are complex, which requires further investigations (19).
Understanding the metabolism of drugs in vivo is a prerequisite for safe, effective and rational drug use, as well as an important means of observing the adverse effects of drugs. In this present study, we focused on measuring the metabolic function of liver and kidneys. We observed that the functional markers of the liver and kidney, including ALT, AST, and urea, decreased in atherosclerotic mice when treated with potassium selencyanoacetate. The liver is an important organ for the site of the catabolism, inactivation, and excretions of many endogenous substances, such as hormones, hemoglobin and metabolites, and exogenous chemicals (such as drugs, poisons), thus making it one of the most vulnerable organs to drug damages. Hematological examinations provide a good understanding of the natures and extents of liver damages, among which, ALT and AST are two most sensitive assays to reflect the existence and the degree of damages in liver cells. The kidney constitutes the principal path for discharging most drugs or their metabolites; thus, renal tissues are readily exposed to drugs and are susceptible to drug damages as well. The assessment of the nephrotoxicity of a new drug is an essential aspect of preclinical research of novel medicines. Although as the main end product of human protein metabolism, urea nitrogen cannot be used as an early functional measurement marker for renal injuries, it is still valuable in the prognosis of the developmental direction of the disease (20). The protective effect of potassium selenium cyanoacetate on liver and kidney function may be related to its antioxidant effect. In future laboratory studies, much attentions should be paid to the underlying mechanisms.
This study has some limitations. Firstly, this study didn’t measure the level of oxidized LDL which is of great significance for the forming atherosclerotic. Secondly, this study didn’t provide mechanisms on how potassium selencyanoacetate reduces the level of TG. The mechanisms underlying this process are complex, which requires further investigation, such as the signaling pathway of this agent reducing the formation of atherosclerotic. This needs to verified by further studies in continuing cell experiments.
Conclusions
A continuous 16-week high-fat diet can successfully generate an ApoE−/− C57BL/6J mouse model of atherosclerosis, and simultaneous administration of high doses of potassium selencyanoacetate (4.63 mg/kg/day) could significantly reduce the TG levels, the formation of aortic plaques and protect the liver and kidney functions in mice fed with a high-fat diet. Therefore, potassium selencyanoacetate probably offers a novel therapeutic in high-fat diet-induced hyperlipidemia, meanwhile may benefit cardiovascular health and prevent ASCVD.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Our study was approved by the Ethics Committee of The Ethics Committee of Animal Protection and Animal Experiment of Wuhan University (Wuhan, China).
References
- Wang YC, McPherson K, Marsh T, et al. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 2011;378:815-25. [Crossref] [PubMed]
- Frostegård J. Immunity, atherosclerosis and cardiovascular disease. BMC Med 2013;11:117. [Crossref] [PubMed]
- Wang Y, Ye L, Ye S, et al. Lipid-lowering and anti-oxidation effects of chondroitin sulfate prepared from squid cartilage in hypercholesterolemia mice. Int J Clin Exp Med 2017;10:2230-40.
- Tang F, Lu M, Zhang S, et al. Vitamin E Conditionally Inhibits Atherosclerosis in ApoE Knockout Mice by Anti-oxidation and Regulation of Vasculature Gene Expressions. LIPIDS 2014;49:1215-23. [Crossref] [PubMed]
- Yang H, Jia X. Safety evaluation of Se-methylselenocysteine as nutritional selenium supplement: Acute toxicity, genotoxicity and subchronic toxicity. Regul Toxicol Pharmacol 2014;70:720-7. [Crossref] [PubMed]
- Yang X, Li J, Hu D, et al. Predicting the 10-Year Risks of Atherosclerotic Cardiovascular Disease in Chinese Population: The China-PAR Project (Prediction for ASCVD Risk in China). Circulation 2016;134:1430-40. [Crossref] [PubMed]
- Liu H, Xu H, Huang K. Selenium in the prevention of atherosclerosis and its underlying mechanisms. Metallomics 2017;9:21-37. [Crossref] [PubMed]
- Ward NC, Watts GF, Eckel RH. Response by Ward et al to Letter Regarding Article, "Statin Toxicity: Mechanistic Insights and Clinical Implications Circ Res 2019;124:e121-2. [PubMed]
- Ali N, Yoshizumi M, Tsuchiya K, et al. Ebselen inhibits p38 mitogen-activated protein kinase-mediated endothelial cell death by hydrogen peroxide. Eur J Pharmacol 2004;485:127-35. [Crossref] [PubMed]
- Ju W, Ji M, Li X, et al. Relationship between higher serum selenium level and adverse blood lipid profile. Clin Nutr 2018;37:1512-7. [Crossref] [PubMed]
- Hasani M, Djalalinia S, Sharifi F, et al. Effect of Selenium Supplementation on Lipid Profile: A Systematic Review and Meta-Analysis. Horm Metab Res 2018;50:715-27. [Crossref] [PubMed]
- Kinoshita M, Yokote K, Arai H, et al. Committee for Epidemiology and Clinical Management of Atherosclerosis. Japan Atherosclerotic Society (JAS) guidelines for prevention of atherosclerotic cardiovascular disease 2017. J Atheroscler Thromb 2018;25:846-984. [Crossref] [PubMed]
- Do R, Willer CJ, Schmidt EM, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet 2013;45:1345-52. [Crossref] [PubMed]
- Ohmura H. Triglycerides as Residual Risk for Atherosclerotic Cardiovascular Disease. Circ J 2019;83:969-70. [Crossref] [PubMed]
- Thomsen M, Varbo A, Tybjaerg-Hansen A, et al. Low nonfasting triglycerides and reduced all-cause mortality: a mendelian randomization study. Clin Chem 2014;60:737-46. [Crossref] [PubMed]
- Johansen CT, Hegele RA. Using Mendelian randomization to determine causative factors in cardiovascular disease. J Intern Med 2013;273:44-7. [Crossref] [PubMed]
- Jørgensen AB, Frikke-Schmidt R, West AS, et al. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J 2013;34:1826-33. [Crossref] [PubMed]
- Pal S, Semorine K, Watts GF, et al. Identification of lipoproteins of intestinal origin in human atherosclerotic plaque. Clin Chem Lab Med 2003;41:792-5. [Crossref] [PubMed]
- Peng J, Luo F, Ruan G, et al. Hypertriglyceridemia and atherosclerosis. Lipids Health Dis 2017;16:233. [Crossref] [PubMed]
- Weiner ID, Mitch WE, Sands JM. Urea and Ammonia Metabolism and the Control of Renal Nitrogen Excretion. Clin J Am Soc Nephrol 2015;10:1444-58. [Crossref] [PubMed]


