
MicroRNA-532-5p protects against atherosclerosis through inhibiting vascular smooth muscle cell proliferation and migration
Introduction
Atherosclerosis (AS) is a specific form of arteriosclerosis, leading to a decline in productivity and a rise in mortality (1,2). AS is characterized by endothelial cell dysfunction, macrophage foam cell formation, and smooth muscle cell (SMC) migration and proliferation (3). Individuals with hypertension history and family history of illness are more vulnerable to AS, especially men (4). AS is generally asymptomatic until it becomes severe enough to cause ischemia even future coronary events, thus early diagnosis and intervention of asymptomatic AS patients are crucial for the disease prevention (5). The long-term expansion of SMCs and arterial endothelial cells have been proved to participate in AS (6). It is well established that abnormal proliferation and migration of human vascular smooth muscle cells (VSMCs) play an important role in the progression of AS (7).
MicroRNAs (miRNAs) is a type of highly conserved, functional non-coding RNAs, regulating protein translation via targeting the target message RNA (mRNA) (8). It is widely reported that miRNA is aberrantly expressed in various human diseases, and their diagnostic and prognostic values attract more and more attention (9-11). It is well known that miRNAs participate in a number of biological processes, including angiogenesis or vascular remodeling, inflammation and oxidative stress, which play a crucial role in the progression of AS (12,13). For example, miR-92a is proved to regulate the activation of endothelial cell, and overexpression of miR-92a promotes the development of AS (14). miR-155 is also reported to promote AS through enhancing vascular inflammation (15). In contrast, miR-30c and miR-126-5p are proved to protect against with AS through regulating endothelial cell proliferation and lipid synthesis (16,17). Notably, the dysregulation of serum miR-532-5p has been identified in patients with ischemic stroke (18). And AS has been reported to be one of the most common causes of ischemic stroke, suggesting the potential role of miR-532-5p in the progress of AS (19). However, its accurate role and detailed mechanism in the development of AS have not been reported.
Therefore, in the present study, we evaluated the expression level of miR-532-5p in AS patients and explored its diagnostic potential in AS. Furthermore, we analyzed the regulating effect of miR-532-5p on the biological behaviors of VSMCs.
Methods
Study population and sample collection
The protocols of the present study were approved by the Ethics Committee of Yidu Central Hospital of Weifang, and all participants involved in the study were asked to sign informed consent.
A total of 180 individuals were recruited in the present study, including 103 patients who were diagnosed with asymptomatic AS and 77 healthy controls. The carotid intima-media thickness (CIMT) of the common carotid artery of each participant was measured using ATL HDI 3000 ultrasound system (Advanced Technology Laboratories, Bothell, WA, USA) equipped with a 5 MHz linear array transducer (20,21). According to the values of CIMT, cases with CIMT ≥0.9 but <1.2 mm were identified as asymptomatic AS (22). Demographic and clinical data of all subjects were recorded, and participants who had the history of clinical macrovascular disease, cardiovascular and cerebrovascular diseases, diabetes mellitus, cancer, smoking, neck surgery, rheumatic immune disease or relevant medical treatment were excluded from the present study. Peripheral blood plasma was collected from each participant after an overnight fast, then the serum was isolated and stored at –80 °C for standby application.
Cell culture and transfection
Human VSMCs were acquired from the American Type Culture Collection (ATCC), and cultured using the Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS, PAN, Aidenbach, Germany) at 37 °C.
The miR-532-5p mimic and inhibitor, or their negative controls (mimic NC or inhibitor NC) were synthesized by RiboBio (Guangzhou, China), Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) was applied for the transfection according to the manufacturer’s instruction. At 48 h post-transfection, transfected VSMCs were collected for further experiment.
RNA extraction and quantitative real-time PCR (qRT-PCR)
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was used for the extraction of total cell and serum RNA, then the RNA was reversely transcribed into cDNA by using Mir-X miRNA First-Strand Synthesis Kit (Takara, Tokyo, Japan). qRT-PCR was performed on the ABI 7300 real-time PCR system (Applied Biosystems, Waltham, MA, USA) with a SYBR green I Master Mix Kit (Invitrogen, Carlsbad, CA, USA). The following thermocycling conditions were used for the PCR: Initial denaturation at 95 °C for 5 min; 30 cycles of 95 °C for 30 sec, 60 °C for 30 sec and 72 °C for 20 sec; and a final extension at 72 °C for 10 min. Cel-miR-39 and U6 were used as an internal control (23). The relative expression of miR-532-5p was calculated based on the 2–ΔΔCt method. The primers used were as follows: miR-532-5p forward, 5'-GCCCATGCCTTGAGTGTAG-3' and reverse, 5'-GTGCGTGTCGTGGAGTCG-3'; and U6 forward, 5'-CTCGCTTCGGCAGCACA-3' and reverse, 5'-AACGCTTCACGAATTTGCGT-3'.
CCK-8 assay
The CCK-8 assay was used for the calculation of cell proliferation. At 48 h post-transfection, transfected VSMCs were seeded into a 96-well plate with the density of 5×103 cells/well and cultured for 3 days continuously. The cell proliferation capacity was detected at 0, 24, 48 and 72 h. Prior to detection, 10 µL CCK-8 solution (Beyotime, Beijing, China) was added to each well, and the optical density (OD) values at the wavelength of 450 nm were detected using a microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).
Transwell migration assay
The treated cells with the density of 5×103 were seeded into the Transwell inserts of the upper chamber (8-µm pore size; BD Biosciences, San Jose, CA, USA) with serum-free DMEM. And the lower chamber was added with DMEM medium with 10% FBS as the attractant. The Transwell plates were incubated in a humidified incubator with 5% CO2 at 37 °C for 48 h, the migrated cells in the bottom side were stained with 0.1% crystal violet for 20 min. The number of migrated cells was counted under an inverted microscope (Olympus Corporation, Tokyo, Japan).
Luciferase reporter assay
PDCD4 was identified to be a candidate target gene of miR-532-5p according to the TargetScan analysis results. Then the luciferase reporter assay was conducted to verify the prediction. The wide type (Wt)-PDCD4 and mutant (Mut)-PDCD4 3'-UTR was cloned into pGL3 luciferase vectors (Promega, Madison, Wisconsin, USA). Then the Wt or Mut reporter vectors and miR-532-5p mimic, miR-532-5p inhibitor or mimic NC, inhibitor NC were co-transfected into human VSMCs. Forty-eight hours post incubation, the luciferase activities were calculated through a dual luciferase assay kit (Promega, Madison, WI, USA). Renilla luciferase was used for normalization.
Statistical analysis
Data were presented as the mean ± SD. All statistical analysis was performed using SPSS 21.0 software (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 7.0 software (GraphPad Software, Inc., USA). The normality of the data was detected by using Kolmogorov-Smirnov (K-S) normality test. According to the test results, mean ± SD was used to express the normal distribution data, and median and interquartile range (IQR) were used to express the non-normal distribution data. Then differences between two groups were compared by using the Mann-Whitney U test for non-normally distributed continuous variables, Student’s t-test for normally distributed continuous variables, chi-squared test for categorical variables. One-way analysis of variance (ANOVA) analysis was applied for the comparison of differences among multiple groups. Correlations between continuous variables were assessed using the Pearson correlation coefficient. A receiver operating characteristic (ROC) analysis was counted to assess the diagnostic value of miR-532-5p in AS, and the area under the curve (AUC) was calculated. P<0.05 was considered to be statistically significant.
Results
Demographic and clinical data
The demographic and clinical data of all participates were recorded in Table 1. It was noted that no significant difference was detected for age, gender, body mass index (BMI), total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglyceride, heart rate, systolic blood pressure (SBP) and diastolic blood pressure (DBP) in AS patients compared with the control group (all P>0.05). Notably, the C-reactive protein (CRP) and CIMT levels were significantly higher in the AS group than healthy controls (P<0.001).
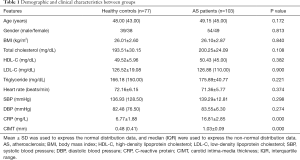
Full table
Serum miR-532-5p levels in AS patients
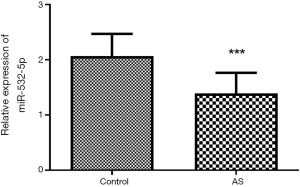
Serum level of miR-532-5p was measured using qRT-PCR. We found that miR-532-5p was downregulated in AS patients compared with that in healthy controls (Figure 1, P<0.001), suggesting the crucial role of miR-532-5p in AS progression.
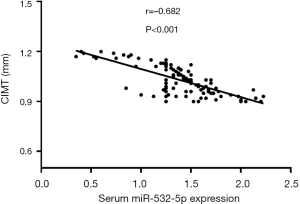
Correlation between miR-532-5p with CIMT in AS patients
CIMT is widely used in the diagnosis of patients with subclinical AS or AS (24,25). Thus, the correlation of miR-532-5p with CIMT was analyzed in AS patients. It was suggested that serum miR-532-5p was inversely related to the CIMT (r=–0.682, P<0.001, Figure 2) in AS patients. We concluded that miR-532-5p might be involved in the development of AS.
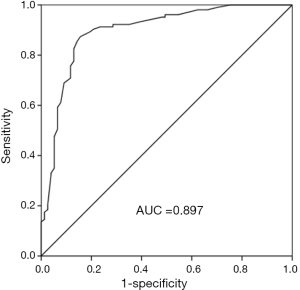
Diagnostic value of miR-532-5p in the patients with AS
Clinically, the diagnostic value of miR-532-5p was further explored. A ROC curve was conducted with an AUC of 0.897, with the sensitivity of 87.4% and the specificity of 84.4% at a cutoff of 1.738, suggesting the high diagnostic accuracy of miR-532-5p in AS patients (Figure 3).
miR-532-5p regulates VSMCs proliferation and migration
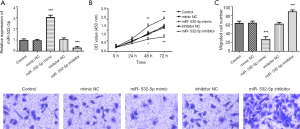
It is well established that abnormal proliferation and migration of human VSMCs are involved in the progression of AS. Here, we proceeded to confirm whether miR-532-5p regulates VSMCs proliferation and migration. As shown in Figure 4A, the miR-532-5p expression level was significantly increased via miR-532-5p mimic transfection compared with the control group, which were markedly decreased after miR-532-5p inhibitor transfection in VSMCs (P<0.001). And the CCK8 assay indicated that miR-532-5p mimic significantly inhibited the cell proliferation, while miR-532-5p inhibitor promoted the cell proliferation markedly (Figure 4B, P<0.001). Furthermore, the cell migration potentials of VSMCs were also determined by the Transwell migration assay. It was observed that overexpression of miR-532-5p by miR-532-5p mimics transfection decreased the number of migrated cells, which were increased by downregulation of miR-532-5p (Figure 4C, P<0.001). These data implicated that overexpression of miR-532-5p inhibited cell proliferation and migration in VSMCs.
PDCD4 is a target gene of miR-532-5p
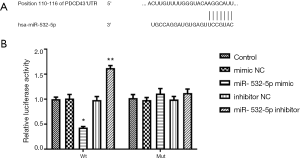
According to the TargetScan results, the binding site of the 3'-UTR of PDCD4 with miR-532-5p was shown in Figure 5A. According to the luciferase reporter gene assay results (Figure 5B), miR-532-5p mimic transfection significantly decreased the luciferase activity of PDCD4, while miR-532-5p inhibitor transfection increased the luciferase activity of PDCD4. However, there was no significant difference for the luciferase activity among groups when the mutant PDCD4 was expressed (Figure 5B).
Discussion
AS is reported to be the main cause of vascular diseases globally, including stroke, arterial disease, and ischemic heart disease. Many risk factors have been identified to be associated with the onset of AS, such as smoking, obesity, hypertension, diabetes and so on (26). The functional disorder of the endothelium and arterial vasculature are involved in the progression of AS. Recently, various miRNAs have been suggested to play a crucial role in the pathogenesis of AS. As Huang et al. reported, higher level of miR-29a was observed in patients with increased CIMT, and increased miR-29a was proved to have good predictive value for an early stage of AS (22). MiR-150 was proved to regulate endothelial cell apoptosis, which plays an important role in the initiation and progression of AS, suggesting the therapeutic potential of miR-150 for endothelial dysfunction and AS (27). In vitro experiment, overexpression of miR-365 suppressed cell proliferation and migration of VSMCs, and miR-365 may influence neointimal formation in AS patients (28).
miR-532-5p is located on human chromosome Xp11.23 with the length of 22 nucleotides, and the sequence of mature miR-532-5p was conserved between most species, suggesting its crucial role in evolutional progress (29). As Li et al. reported, miR-532-5p is aberrantly expressed in patients with ischemic stroke, and might be a potential diagnostic biomarker in ischemic stroke, which is closely related to the occurrence of AS (18). A recent study also suggested that serum miR-532-5p level was downregulated in intracerebral hemorrhage (ICH) patients compared with the vascular risk factor controls (VRFCs), who were diagnosed with hypertension, hyperlipidemia, atrial fibrillation or diabetes, but not having a prior stroke, myocardial infarction, or transient ischemic attack (TIA) (30). Expectedly, the present results indicated that serum miR-532-5p level was markedly decreased in AS patients compared with healthy controls, suggesting the potential role of miR-532-5p in the progression of AS.
CIMT is a widely used surrogate marker for AS worldwide, which is generally considered to be an important index to predict the progression of AS (24,25). In the presents study, serum miR-532-5p was found to be inversely related to the CIMT in AS patients. Furthermore, a ROC curve was conducted to assess the diagnostic value of miR-532-5p in AS, which indicated that miR-532-5p was a potential diagnostic biomarker with relatively high sensitivity and specificity. The diagnostic value of miRNAs in AS has been widely reported. For example, miR-29b was overexpressed in patients with higher CIMT, and showed a positive association with CIMT, suggesting that miR-29b might be a novel biomarker for the identification of AS (31). Additionally, in patients with essential hypertension, circulating miR-92a was proved to be a potential noninvasive AS marker (32). In the present study, the decreased expression of miR-532-5p was indicated to be a novel potential biomarker in AS.
The regulating effect of miR-532-5p in cell proliferation and migration has been widely reported. For example, downregulation of miR-532-5p was reported to inhibit bladder cancer cell proliferation and invasion (33). In the study of ovarian cancer, miR-532-5p was also suggested to suppress cell progression by targeting TWIST1 (34). It is well established that abnormal proliferation and migration of human VSMCs are involved in the progression of AS. And various miRNAs have been reported to be involved in the regulation of VSMCs proliferation and migration, such as miR-448, miR-379, miR-133b (35-37). In the present study, in vitro function experiments suggested that overexpression of miR-532-5p inhibited the proliferation and migration of VSMCs, which was promoted by the downregulation of miR-532-5p. All evidence suggested the inhibiting effect of miR-532-5p in the biological behaviors of VSMCs, which might be the potential mechanism of the role miR-532-5p in AS. Further studies are warranted to explore the molecular mechanisms of miR-532-5p in AS.
It is well known that miRNA functions through targeting mRNA to inhibit translation (38). In a study about the mechanism of acute myocardial infarction, programmed cell death protein 4 (PDCD4) was identified to be the target gene of miR-532-5p (39). Additionally, in a rat model of coronary AS, PDCD4 was proved to be highly expressed in the coronary artery tissues, suggesting its potential role in the formation of coronary AS plaque (40). In the present study, the luciferase reporter gene assay results indicated that PDCD4 was the target gene of miR-532-5p in human VSMCs. Furthermore, PDCD4 was suggested to regulate proliferation and apoptosis of SMCs during the plaque formation by previous evidence (40,41). Considering the evidence, we speculated that miR-532-5p might participate in the pathological processes of AS through targeting PDCD4. However, further studies are needed for the further exploration of the mechanism of miR-532-5p with the involvement of PDCD4 in AS.
In conclusion, we demonstrated that miR-532-5p was downregulated in patients with AS, and decreased expression of miR-532-5p might be a potential diagnostic biomarker for AS. Overexpression of miR-532-5p inhibited the proliferation and migration of VSMCs. The present results provided evidence about the involvement of miR-532-5p in the pathogenesis of AS, and indicated a therapeutic potential of miR-532-5p for AS. And targeting miR-532-5p provides new insight into understanding abnormal biological behavior of VSMCs, which is involved in the progression of AS.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-91). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The protocols of the present study were approved by the Ethics Committee of Yidu Central Hospital of Weifang (No. 20170225), and all participants involved in the study were asked to sign informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med 2011;17:1410-22. [Crossref] [PubMed]
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 2011;473:317-25. [Crossref] [PubMed]
- Jain T, Nikolopoulou EA, Xu Q, et al. Hypoxia inducible factor as a therapeutic target for atherosclerosis. Pharmacol Ther 2018;183:22-33. [Crossref] [PubMed]
- Buttari B, Profumo E, Rigano R. Crosstalk between red blood cells and the immune system and its impact on atherosclerosis. Biomed Res Int 2015;2015:616834.
- Miller M. An emerging paradigm in atherosclerosis: focus on subclinical disease. Postgrad Med 2009;121:49-59. [Crossref] [PubMed]
- Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 2013;13:709-21. [Crossref] [PubMed]
- Zheng J, Chen K, Wang H, et al. SIRT7 regulates the vascular smooth muscle cells proliferation and migration via Wnt/beta-catenin signaling pathway. Biomed Res Int 2018;2018:4769596.
- An JX, Ma ZS, Ma MH, et al. MiR-1236-3p serves as a new diagnostic and prognostic biomarker for gastric cancer. Cancer Biomark 2019;25:127-32. [Crossref] [PubMed]
- Sun R, Muheremu A, Hu Y. miRNA-30c can be used as a target in the diagnosis and treatment of osteosarcoma. Onco Targets Ther 2018;11:9091-9. [Crossref] [PubMed]
- Yang ZQ, Wu CA, Cheng YX. Prognostic value of microRNA-133a expression and its clinicopathologic significance in non-small cell lung cancer: a comprehensive study based on meta-analysis and the TCGA database. Oncol Res Treat 2018;41:762-8. [Crossref] [PubMed]
- Liu J, Chen Z, Xiang J, et al. MicroRNA-155 acts as a tumor suppressor in colorectal cancer by targeting CTHRC1 in vitro. Oncol Lett 2018;15:5561-8. [PubMed]
- Fang YC, Yeh CH. Role of microRNAs in vascular remodeling. Curr Mol Med 2015;15:684-96. [Crossref] [PubMed]
- Huo L, Wang B, Zheng M, et al. miR-128-3p inhibits glioma cell proliferation and differentiation by targeting NPTX1 through IRS-1/PI3K/AKT signaling pathway. Exp Ther Med 2019;17:2921-30. [PubMed]
- Loyer X, Potteaux S, Vion AC, et al. Inhibition of microRNA-92a prevents endothelial dysfunction and atherosclerosis in mice. Circ Res 2014;114:434-43. [Crossref] [PubMed]
- Nazari-Jahantigh M, Wei Y, Noels H, et al. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J Clin Invest 2012;122:4190-202. [Crossref] [PubMed]
- Soh J, Iqbal J, Queiroz J, et al. MicroRNA-30c reduces hyperlipidemia and atherosclerosis in mice by decreasing lipid synthesis and lipoprotein secretion. Nat Med 2013;19:892-900. [Crossref] [PubMed]
- Schober A, Nazari-Jahantigh M, Wei Y, et al. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med 2014;20:368-76. [Crossref] [PubMed]
- Li P, Teng F, Gao F, et al. Identification of circulating microRNAs as potential biomarkers for detecting acute ischemic stroke. Cell Mol Neurobiol 2015;35:433-47. [Crossref] [PubMed]
- Farré R, Rotger M, Montserrat JM, et al. Analog circuit for real-time computation of respiratory mechanical impedance in sleep studies. IEEE Trans Biomed Eng 1997;44:1156-9. [Crossref] [PubMed]
- Cardellini M, Marini MA, Frontoni S, et al. Carotid artery intima-media thickness is associated with insulin-mediated glucose disposal in nondiabetic normotensive offspring of type 2 diabetic patients. Am J Physiol Endocrinol Metab 2007;292:E347-52. [Crossref] [PubMed]
- Polak JF, Pencina MJ, Meisner A, et al. Associations of carotid artery intima-media thickness (IMT) with risk factors and prevalent cardiovascular disease: comparison of mean common carotid artery IMT with maximum internal carotid artery IMT. J Ultrasound Med 2010;29:1759-68. [Crossref] [PubMed]
- Huang YQ, Cai AP, Chen JY, et al. The relationship of plasma miR-29a and oxidized low density lipoprotein with atherosclerosis. Cell Physiol Biochem 2016;40:1521-8. [Crossref] [PubMed]
- Kroh EM, Parkin RK, Mitchell PS, et al. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR). Methods 2010;50:298-301. [Crossref] [PubMed]
- Nezu T, Hosomi N, Aoki S, et al. Carotid intima-media thickness for atherosclerosis. J Atheroscler Thromb 2016;23:18-31. [Crossref] [PubMed]
- Kurkowska-Jastrzebska I, Karlinski MA, Blazejewska-Hyzorek B, et al. Carotid intima media thickness and blood biomarkers of atherosclerosis in patients after stroke or myocardial infarction. Croat Med J 2016;57:548-57. [Crossref] [PubMed]
- Zmysłowski A, Szterk A. Current knowledge on the mechanism of atherosclerosis and pro-atherosclerotic properties of oxysterols. Lipids Health Dis 2017;16:188. [Crossref] [PubMed]
- Qin B, Shu Y, Xiao L, et al. MicroRNA-150 targets ELK1 and modulates the apoptosis induced by ox-LDL in endothelial cells. Mol Cell Biochem 2017;429:45-58. [Crossref] [PubMed]
- Kim MH, Ham O, Lee SY, et al. MicroRNA-365 inhibits the proliferation of vascular smooth muscle cells by targeting cyclin D1. J Cell Biochem 2014;115:1752-61. [Crossref] [PubMed]
- Xu X, Zhang Y, Liu Z, et al. miRNA-532-5p functions as an oncogenic microRNA in human gastric cancer by directly targeting RUNX3. J Cell Mol Med 2016;20:95-103. [Crossref] [PubMed]
- Cheng X, Ander BP, Jickling GC, et al. MicroRNA and their target mRNAs change expression in whole blood of patients after intracerebral hemorrhage. J Cereb Blood Flow Metab 2020;40:775-86. [Crossref] [PubMed]
- Huang YQ, Li J, Chen JY, et al. The association of circulating miR-29b and interleukin-6 with subclinical atherosclerosis. Cell Physiol Biochem 2017;44:1537-44. [Crossref] [PubMed]
- Huang Y, Tang S, Ji-Yan C, et al. Circulating miR-92a expression level in patients with essential hypertension: a potential marker of atherosclerosis. J Hum Hypertens 2017;31:200-5. [Crossref] [PubMed]
- Xie X, Pan J, Han X, et al. Downregulation of microRNA-532-5p promotes the proliferation and invasion of bladder cancer cells through promotion of HMGB3/Wnt/beta-catenin signaling. Chem Biol Interact 2019;300:73-81. [Crossref] [PubMed]
- Wei H, Tang QL, Zhang K, et al. miR-532-5p is a prognostic marker and suppresses cells proliferation and invasion by targeting TWIST1 in epithelial ovarian cancer. Eur Rev Med Pharmacol Sci 2018;22:5842-50. [PubMed]
- Zhang R, Sui L, Hong X, et al. MiR-448 promotes vascular smooth muscle cell proliferation and migration in through directly targeting MEF2C. Environ Sci Pollut Res Int 2017;24:22294-300. [Crossref] [PubMed]
- Li K, Wang Y, Zhang A, et al. miR-379 inhibits cell proliferation, invasion, and migration of vascular smooth muscle cells by targeting insulin-like factor-1. Yonsei Med J 2017;58:234-40. [Crossref] [PubMed]
- Liu H, Xiong W, Liu F, et al. MicroRNA-133b regulates the growth and migration of vascular smooth muscle cells by targeting matrix metallopeptidase 9. Pathol Res Pract 2019;215:1083-8. [Crossref] [PubMed]
- Jin X, Wang Z, Pang W, et al. Upregulated hsa_circ_0004458 contributes to progression of papillary thyroid carcinoma by inhibition of miR-885-5p and activation of RAC1. Med Sci Monit 2018;24:5488-500. [Crossref] [PubMed]
- Ma J, Zhang J, Wang Y, et al. MiR-532-5p alleviates hypoxia-induced cardiomyocyte apoptosis by targeting PDCD4. Gene 2018;675:36-43. [Crossref] [PubMed]
- Gao Y, Li H, Zhou Y, et al. PDCD4 expression in coronary atherosclerosis rat models and its mechanism. Exp Ther Med 2019;17:3150-4. [PubMed]
- Yu X, Li Z. MicroRNAs regulate vascular smooth muscle cell functions in atherosclerosis Int J Mol Med 2014;34:923-33. (review). [Crossref] [PubMed]


