
An analysis of the correlation between the human apolipoprotein E gene polymorphism and lipoprotein-associated phospholipase A2
Introduction
Atherosclerosis is one of the most common cardiovascular and cerebrovascular diseases (1). With the change of people’s lifestyle, the mortality rate of atherosclerosis has increased yearly (2) and even if patients are treated in time, their quality of life will be greatly affected by the disease (3).
There are many causes of atherosclerosis, among which genes and heredity are important factors (4). Apolipoprotein E (ApoE) is the structural protein of many kinds of lipoproteins, it is particularly important to note that ApoE is one of the main components of low density lipoprotein (LDL) (5), therefore, it plays an important role in the process of plasma lipid transport and metabolism and can affect the level of blood lipid and the formation of atherosclerotic lesions in varying degrees (6). The ApoE gene, located on chromosome 19, has obvious genetic polymorphism (7). There are three isomers of ApoE: E2, E3, and E4, which are encoded by three alleles: ε 2, ε 3, and ε 4 that are located at a single locus on chromosome 19. ApoE has six phenotypes, namely three homozygotes (E2/2, E3/3, E4/4) and three heterozygotes (E2/3, E2/4, E3/4) and the molecular basis is the mutual substitution of cysteine and arginine: ε 2 contains two cysteines, ε 3 is due to the replacement of one of the cysteines by arginine, ε 4 is due to the replacement of both the cysteines by arginines (8). These three isomers and six phenotypes of ApoE affect lipid metabolism due to different affinities to receptors, which accordingly affect the level of blood lipid and the formation of atherosclerotic lesions in varying degrees (9).
Plasma lipoprotein-associated phospholipase A2 (Lp-PLA2), also known as platelet activating factor acetylhydrolase (PAF-AH), is mainly synthesized and secreted by the inflammatory cells in atherosclerotic plaque, such as mature macrophages and lymphocytes, 80% of which is bound to LDL (10). Lp-PLA2 can oxidize and modify LDL by removing oxidized phosphatidylcholine on the surface of LDL (11), thereby produces lysophosphatidylcholine (lyso-PC) that can promote a series of endothelial inflammatory reactions (12). A previous study revealed that, as a new inflammatory marker, Lp-PLA2 was involved in all stages of atherosclerotic plaque formation (13).
Although many studies have respectively revealed that ApoE and Lp-PLA2 are involved in the occurrence and development of atherosclerosis, there are few studies on the association between ApoE gene polymorphism and Lp-PLA2, which, however, was analyzed in the present study.
Methods
Study subjects
A total of 413 patients who visited the People’s Hospital in Qingyuan City from June 2016 to June 2019 were randomly selected. There were 220 patients in the atherosclerotic cardiovascular and cerebrovascular disease group (experimental group). The inclusion criteria were patients with atheromatous plaques, including cerebral infarction, cerebrovascular accident, stroke, coronary heart disease or other types of atherosclerotic cardiovascular and cerebrovascular diseases. The exclusion criteria were patients with cardiac insufficiency. Among these patients, 130 were male and 90 were female and the age of these patients ranged within 29–91 years old, with an average age of 66.8±11.8 years old. There were 193 patients in the non-atherosclerotic cardiovascular and cerebrovascular disease group (control group) and all these patients came from the outpatients in the same period as that of the atherosclerotic cardio-cerebrovascular disease group. These patients had no stroke confirmed by computed tomography (CT)/magnetic resonance imaging (MRI). Among these patients, 107 were male and 86 were female and the age of these patients ranged within 30–93 years old, with an average age of 58.0±13.6 years old. This study was conducted in accordance with the declaration of Helsinki and approved by the Ethics Committee of The Sixth Affiliated Hospital of Guangzhou Medical University & Qingyuan People’s Hospital. All patients provided signed informed consent.
Study methods
Specimen collection
At 12–14 hours after fasting, 4 mL of the elbow vein blood was withdrawn and sub-packed, 2 mL of it was placed in an EDTA anticoagulation tube and centrifuged at 1,500×g for 10 minutes, then the supernatant was collected and sent for testing for Lp-PLA2; the remaining 2 mL of it was placed in an EDTA anticoagulation tube and preserved at −20 °C for detecting ApoE genotypes.
Detection of ApoE gene polymorphism
A Lab-Aid 824 nucleic acid extraction Mini kit (Xiamen Zeesan Biotechnology Co., Ltd.) was used to extract DNA according to the reagent instructions. An ApoE gene detection kit (Wuhan Youzhiyou Medical Technology Co., Ltd.) was used to amplify the target gene on a Cfx-96 fluorescence quantitative polymerase chain reaction (PCR) instrument (bole, USA) and detect its fluorescence intensity. Primer sequences: upstream primer: 5'-ACAGAATTCGCCGGCCCTGGTAC-3', downstream primer: 5'-TAAGCTI'GGCACGCZTGTCCAAGGA-3'. The reaction system included: PCR buffer, dNTPs, specific primers and probes, internal standard primers and probes, Taq enzyme, UNG enzyme and the total volume was 25 µL. The reaction conditions of cDNA amplification via PCR were as follows: treated with Uracil-N-glycosylase (UNG) at 37 °C for 10 minutes, then pre-denatured at 95 °C for 5 minutes and at 95 °C for 15 seconds, then at 60 °C for 60 seconds, and the fluorescence signal was collected at the end of this stage, a total of 40 cycles were performed. Finally, the CFX Manager software was used to analyze the fluorescence intensity curve.
Detection of the plasma Lp-PLA2 level
An Lp-PLA2 quantitative assay kit for human plasma was used and the level of Lp-PLA2 in plasma was detected by enzyme-linked immunosorbent assay (ELISA). The normal reference values are as follows: <175 ng/mL is normal and >175 ng/mL is abnormal (14).
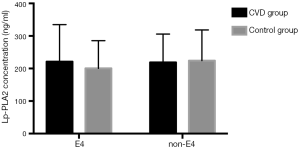
Analysis of Lp-PLA2 concentration distribution difference between ApoE E4 and non-E4 in both experimental group and control group
The phase diagram of Lp-PLA2 concentration distribution between ApoE E4 and non-E4 in the experimental and control group is presented in Figure 1. In the control group, the median of Lp-PLA2 in patients with allele E4 was 121.56 ng/mL and the median of Lp-PLA2 in patients without allele E4 was 198.5 ng/mL, the difference was not statistically significant (t=−0.97, P=0.3356); in the experimental group, the median of Lp-PLA2 in patients with allele E4 was 182.3 ng/mL and the median of Lp-PLA2 in patients without allele E4 was 186.4 ng/mL, the difference was not statistically significant (t=−0.16, P=0.8707).
Statistical analysis
All data were processed using statistical software SPSS 22.0. Measurement data were expressed as mean ± standard deviation (x ± SD). The intergroup comparison was conducted using an analysis of variance. The comparison of genotype proportion and allele frequency between the groups was conducted using an χ2 test.
Results
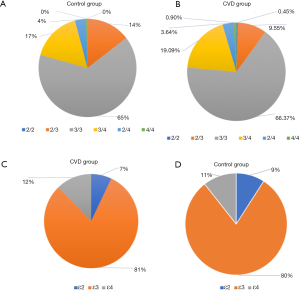
Comparison of ApoE genotype and allele between the experimental group and the control group
As shown in Figure 2, in the experimental group, the frequency of ApoE genotype distribution was E3/3 (66.36%) > E3/4 (19.09%) > E2/3 (9.55%) > E2/4 (3.64%) > E4/4 (0.9%) > E2/2 (0.45%); and in the control group, it was E3/3 (64.77%) > E3/4 (16.58%) > E2/3 (14.51) > E2/4 (1.16%) > E4/4 (0.52%). The E3/3 genotype was the most common in both groups. The frequencies of ApoE alleles were 80.68% and 80.31% (ε 3), 12.27% and 10.62% (ε 4), and 7.05% and 9.07% (ε 2) in the experimental and control groups. The difference in genotype proportion (χ2=329, P=0.604) and allele frequency (χ2=1.559, P=0.459) between the two groups were not statistically significant.
Comparison of the Lp-PLA2 level among patients with different genotypes in the experimental group
As shown in Figure 3, the level of Lp-PLA2 in patients with different genotypes in the experimental group was as follows: 223.1±96.7 ng/mL (E2/3), 218.6±86.0 ng/mL (E3/3), E3/4 227.2±118.2 ng/mL (E3/4), 187.6±103.6 ng/mL (E2/4), and 230.6±6.9 ng/mL (E4/4), respectively. An analysis of variance revealed that the difference in the Lp-PLA2 level among the genotypes was not statistically significant (P>0.05).
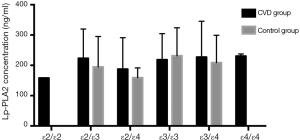
Comparison of the Lp-PLA2 level in patients with different genotypes in the control group
As shown in Figure 3, in control group, the level of Lp-PLA2 was as follows: 194.8±100.5 ng/mL (E2/3), 231.4±92.3 ng/mL (E3/3), 208.8±90.5 ng/mL (E3/4), and 159.0±32.5 ng/mL (E2/4), respectively and no patient with E4/4 was found. An analysis of variance revealed that the difference in the Lp-PLA2 level among the genotypes was not statistically significant (P>0.05).
Discussion
In view of the fact that the Lp-PLA2 may be related to the formation of atherosclerosis, the purpose of this study was to determine whether ApoE gene polymorphism affects the plasma level of Lp-PLA2, in order to figure out the role of Lp-PLA2 in AS and to provide a reference basis for clinical diagnosis and treatment. A previous study revealed that the distribution of six genotypes of the ApoE gene was uneven in a normal population, the distribution frequency of E3/3 was the highest in the general population, the frequency was mostly more than 60%, the heterozygotes (E2/3, E3/4) with ApoE3 was in the middle, accounting for approximately 30%, the frequency of E2/2, E4/4 and E2/4 was the lowest, and the sum of the three was generally no more than 10%, while the most common allele in the population was ε 3 (15). The results of the present study revealed that in the experimental and control group, the frequency of ApoE3/3 genotype was the highest, accounting for 66.36% and 64.77%, respectively and the frequency of ε3 was also the highest in the alleles of ApoE, accounting for 80.68% and 80.31%, respectively. These findings are consistent with the results of other epidemiological studies.
In the present study, ApoE gene polymorphism was detected in patients in the experimental and control group. The investigators revealed, in comparison, that the differences in gene composition and gene frequency between the two groups were not statistically significant (P>0.05) and could, therefore, not conclude that ApoE gene polymorphism is correlated to atherosclerotic cardiovascular and cerebrovascular diseases. The causes of the results were analyzed and the investigators considered that the reason may be that the most common allele in the population is ε 3, however, the allele ε 4 causing atherosclerosis and ε 2 with an anti-atherosclerosis function has no significant distribution (15). Therefore, it is very difficult to get different results under the limited conditions of the small sample in this study, suggesting that larger patient samples are needed for analysis in the future to get a clearer conclusion.
Currently, there’s no consensus on the role of Lp-PLA2 in AS. Its effects on AS plaques may depend on the type of lipoprotein particles (16,17). In the present study, the plasma level of Lp-PLA2 was also measured. The results revealed that, in both groups, the difference in the level of Lp-PLA2 among different genotypes was not statistically significant (P>0.05), that is to say, it could not conclude that the ApoE gene polymorphism is correlated to Lp-PLA2 level. Previous studies have revealed that Lp-PLA2 released into blood circulation mainly bound to apolipoprotein B-rich lipoproteins and LDL accounted for 80% of them (16,18,19). The level of Lp-PLA2 was positively correlated with the level of LDL subfractions in patients with atherosclerotic diseases (20,21). However, the present study suggests that ApoE gene polymorphism is not necessarily correlated to the Lp-PLA2 level. Therefore, the level of Lp-PLA2 may be correlated to the polymorphism of other components in LDL, such as apolipoprotein B. Since the sample size of this study was small and the individual difference in gene polymorphism is relatively large, a greater patient sample is needed for analysis in the future to get a clearer conclusion.
Conclusions
The differences in gene composition and gene frequency of ApoE gene polymorphism between patients with atherosclerotic and non-atherosclerotic cardiovascular and cerebrovascular diseases were not statistically significant. Therefore, this study could not conclude that ApoE gene polymorphism is correlated to atherosclerotic cardiovascular and cerebrovascular diseases. In both groups the difference in the level of Lp-PLA2 among different genotypes was also not statistically significant, therefore, this study could not conclude that ApoE gene polymorphism is correlated to Lp-PLA2 level.
Acknowledgments
Funding: This work was supported by Qingyuan Industrial Technology Research and Development Special Funds (No.2017A021) and Natural Science Foundation of Guangdong Province (No.2018A030307065).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/cdt-20-43). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the declaration of Helsinki and approved by the Ethics Committee of The Sixth Affiliated Hospital of Guangzhou Medical University & Qingyuan People’s Hospital. All patients provided signed informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Libby P, Bornfeldt KE, Tall AR. Atherosclerosis: Successes, Surprises, and Future Challenges. Circ Res 2016;118:531-4. [Crossref] [PubMed]
- Mohd Sahardi NFN, Makpol S. Ginger (Zingiber officinale Roscoe) in the Prevention of Ageing and Degenerative Diseases: Review of Current Evidence. Evid Based Complement Alternat Med 2019;2019:5054395. [Crossref] [PubMed]
- Choudhary NS, Duseja A. Screening of Cardiovascular Disease in Nonalcoholic Fatty Liver Disease: Whom and How? J Clin Exp Hepatol 2019;9:506-14. [Crossref] [PubMed]
- Yang Z, Wang H, Edwards D, et al. Association of blood lipids, atherosclerosis and statin use with dementia and cognitive impairment after stroke: a systematic review and meta-analysis. Ageing Res Rev 2020;57:100962. [Crossref] [PubMed]
- Getz GS, Reardon CA. Apoprotein E and Reverse Cholesterol Transport. Int J Mol Sci 2018;19:E3479. [Crossref] [PubMed]
- Rebeck GW. The role of APOE on lipid homeostasis and inflammation in normal brains. J Lipid Res 2017;58:1493-9. [Crossref] [PubMed]
- Huebbe P, Rimbach G. Evolution of human apolipoprotein E (APOE) isoforms: Gene structure, protein function and interaction with dietary factors. Ageing Res Rev 2017;37:146-61. [Crossref] [PubMed]
- Abyadeh M, Djafarian K, Heydarinejad F, et al. Association between Apolipoprotein E Gene Polymorphism and Alzheimer's Disease in an Iranian Population: A Meta-Analysis. J Mol Neurosci 2019;69:557-62. [Crossref] [PubMed]
- Abondio P, Sazzini M, Garagnani P, et al. The Genetic Variability of APOE in Different Human Populations and Its Implications for Longevity. Genes (Basel) 2019;10:E222. [Crossref] [PubMed]
- Hu G, Liu D, Tong H, et al. Lipoprotein-Associated Phospholipase A2 Activity and Mass as Independent Risk Factor of Stroke: A Meta-Analysis. Biomed Res Int 2019;2019:8642784. [Crossref] [PubMed]
- Zhu S, Wei X, Yang X, et al. Plasma Lipoprotein-associated Phospholipase A2 and Superoxide Dismutase are Independent Predicators of Cognitive Impairment in Cerebral Small Vessel Disease Patients: Diagnosis and Assessment. Aging Dis 2019;10:834-46. [Crossref] [PubMed]
- Huang L, Yao S. Carotid artery color Doppler ultrasonography and plasma levels of lipoprotein-associated phospholipase A2 and cystatin C in arteriosclerotic cerebral infarction. J Int Med Res 2019;47:4389-96. [Crossref] [PubMed]
- Zalewski A, Macphee C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: biology, epidemiology, and possible therapeutic target. Arterioscler Thromb Vasc Biol 2005;25:923-31. [Crossref] [PubMed]
- Zheng D, Zeng F, Cai A, et al. Baseline elevated Lp-PLA2 is associated with increased risk for re-stenosis after stent placement. Lipids Health Dis 2014;13:41. [Crossref] [PubMed]
- Bian L, Mao LG, Sun Y, et al. Serum lipoprotein-associated phospholipase A2 as a promising prognostic biomarker in association with 90-day outcome of acute intracerebral hemorrhage. Clin Chim Acta 2019;495:429-35. [Crossref] [PubMed]
- Tellis CC, Tselepis AD. Pathophysiological Role and Clinical Significance of Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) Bound to LDL and HDL. Curr Pharm Des 2014;20:6256-69. [Crossref] [PubMed]
- Huang F, Wang K, Shen J. Lipoprotein-associated phospholipase A2: The story continues. Med Res Rev 2020;40:79-134. [Crossref] [PubMed]
- Pokharel Y, Mouhanna F, Nambi V, et al. ApoB, small-dense LDL-C, Lp(a), LpPLA2 activity, and cognitive change. Neurology 2019;92:e2580-93. [Crossref] [PubMed]
- Krebs A, Kratzin T, Doerfer J, et al. Decrease of small dense LDL and lipoprotein-associated phospholipase A2 due to human growth hormone treatment in short children with growth hormone deficiency and small for gestational age status. J Pediatr Endocrinol Metab 2016;29:203-8. [Crossref] [PubMed]
- Garg S, Madhu SV, Suneja S. Lipoprotein associated phospholipase A2 activity & its correlation with oxidized LDL & glycaemic status in early stages of type-2 diabetes mellitus. Indian J Med Res 2015;141:107-14. [Crossref] [PubMed]
- Sönmez D, Fidan Y, Ozcan O, et al. Is there a relationship between small, dense LDL and lipoprotein--associated phospholipase A2 mass in dialysis patients? Clin Lab. 2014;60:1431-7. [Crossref] [PubMed]


