
Cardiogenic shock in an 8-year-old child secondary to anomalous coronary origin and course: a case report on the value of coronary computed tomography angiography and cardiac magnetic resonance
Introduction
Cardiogenic shock is a life-threatening condition resulting from an inadequate circulation due to primary failure of cardiac function. The underlying etiology can include myocardial infarction, cardiomyopathy, myocarditis, congenital heart disease and other conditions. Myocardial infarction is the most common cause in adults, but rare in children (1). Congenital coronary anomalies are the most common cause of myocardial infarction in children (2). Coronary computed tomography angiography (CCTA) has been widely used clinically in the screening and diagnosis of suspected coronary disease in adults. CCTA can be performed with high-quality image and acceptable radiation exposure in children. Cardiac magnetic resonance (CMR) has unique value in the diagnosis of cardiovascular disease. However use of CMR in younger and critically ill patients and children with claustrophobia is limited by long scanning time, noisy environment, and requirements for frequent breath holding.
This case reports an 8-year-old male child with cardiogenic shock caused by acute myocardial infarction, with the left coronary artery originating from the right sinus of Valsalva with and interarterial course. CCTA and CMR played an important role in the diagnosis and assessment. We present the following article in accordance with the CARE reporting checklist. (available at http://dx.doi.org/10.21037/cdt-20-422).
Case presentation
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this study and any accompanying images. An 8-year-old male child was admitted to the emergency department because of sudden dizziness, chest pain, and fatigue when running three hours ago. There was no evidence of convulsion, fever or incontinence. During transportation to the hospital, he repeatedly coughed up pink, foamy sputum in the ambulance. At admission, vital signs were consistent with shock with a blood pressure of 76/62 mmHg and a heart rate of 139 beats/min. Physical examination was further notable for a respiratory rate of 36 breaths/min, and a congested pharynx. Crackles were audible over the bilateral lung lobes. The limbs were slightly cold, with a capillary filling test greater than 3 seconds. Over the past two years, he had two episodes of syncope during exercise, accompanied by incontinence. In both instances he regained consciousness immediately after rest, but his parents did not seek medical attention. Family history was negative for similar syncopal attacks, sudden cardiac arrest, or arrhythmia.
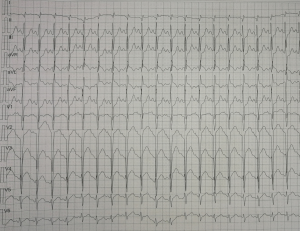
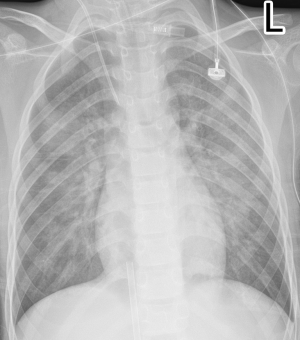
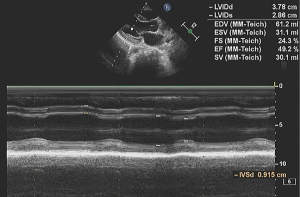
On the first day of admission, laboratory values showed an increased white blood cell count of 17.53×109/L (normal range, 5–12×109/L), neutrophile granulocyte count 9.48×109/L (normal range, 1.80–6.30×109/L) and elevated lactic acid of 3.9 mmol/L (normal range, 0.5–2.0 mmol/L). Blood gases showed evidence of respiratory failure with reduced carbon dioxide pressure of 31.6 mmHg (normal range, 35–45 mmHg), artery oxygen pressure of 29.2 mmHg (normal range, 80–100 mmHg), oxygen saturation of 70.3%, (normal range, 91.9–99.0%). Cardiac enzymes were elevated with a troponin I of 0.53 ng/mL (normal range, 0–0.06 ng/mL), creatine kinase of 230 IU/L (normal range, 39–308 IU/L), creatinine kinase isoenzyme of 38 IU/L (normal range, 0–24 IU/L) and brain natriuretic peptide of 42 pg/mL (normal range, 0–125 pg/mL) (Table 1). The electrocardiogram showed sinus tachycardia with abnormal Q wave (I, AVL and V1-V3 leads) (Figure S1). Bedside chest radiograph showed multiple patchy bilateral opacifications, more pronounced in the perihilar segments, consistent with acute cardiogenic pulmonary edema (Figure S2). On echocardiography, motion of left ventricular apical anterior wall was mildly weakened, left ventricular ejection fraction was reduced (49%) (Figure S3). There was a trivial pericardial effusion. The patient was admitted to intensive care unit on the second day and underwent veno-arterial extracorporeal membrane oxygenation (VA-ECMO) for four days, due to sustained ventricular tachycardia and hypotension.
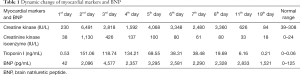
Full table
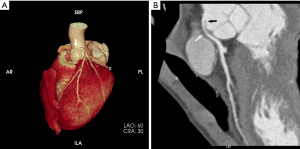
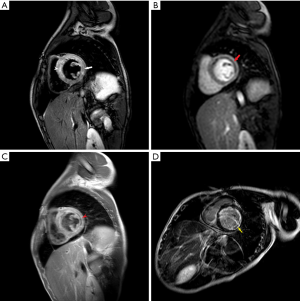
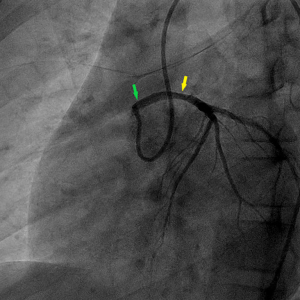
On the ninth day, CCTA was performed after stabilization and discontinuation of VA-ECMO, using a 256-slice CT scanner (Brilliance iCT Philips, Netherlands). CCTA revealed an anomalous left coronary artery originated from the right sinus of Valsalva, with an acute angle at the takeoff and inter-arterial course between aortic root and pulmonary trunk. The mid-portion of left main coronary artery was narrowed by 50%, the anterior descending branch, circumflex branch, and right coronary arteries were normal (Figure 1). On the thirteenth day, he also underwent CMR by a 3.0-T scanner (Achieva, Philips, Netherlands). The motion of basal-, mid-anteroseptal, anterior and lateral wall was reduced. The signal intensity of left ventricular anterior, septal and lateral wall from base to apex was heterogeneously increased on T2 weighted image, indicated myocardial edema. Moderately high signal was noted in endocardial and mid-myocardial layers, a finding seen with intramyocardial hemorrhage (IMH). Rest myocardial perfusion showed an endocardial perfusion defect in the basal-, mid-anteroseptal, anterior and lateral wall. Early gadolinium enhancement sequences showed low signal intensity basal-lateral, and mid-anteroseptal wall. Late gadolinium enhancement (LGE) sequences showed endocardial enhancement in left ventricular anterior, septal and lateral wall. Low signal intensity in LGE region was seen in basal-lateral and mid-anteroseptal wall, indicated microvascular obstruction (MVO) (Figure 2). An extracellular volume value of 45% was observed with T1 mapping. The calculated EF (59%) indicated preserved global left ventricular function, but global circumferential strain and longitudinal strain were reduced. On the fifteenth day, coronary angiography confirmed the diagnosis of anomalous origin and left main coronary artery stenosis (40%) (Figure 3).
The patient remained clinically stable and chest pain free for one week, and he was discharged home, with a strong recommendation to avoid exercise. The coronary artery anomaly was corrected surgically one month later because of recurrent chest pain. Followed up over four months was unremarkable without chest pain or major adverse cardiovascular events (MACE).
Discussion
The causes of cardiogenic shock in children include fulminant myocarditis, myocardial infarction, congenital heart disease, cardiomyopathy, drug abuse, and other conditions (3). Acute myocardial infarction is rare in children. Myocardial infarction in children can be caused by congenital coronary artery anomalies, but also by Kawasaki disease, coronary artery thrombosis and coronary spasm (2,4-6). Congenital coronary artery anomalies are the most common etiology, with an incidence of 0.3–1% (7). Most coronary artery anomalies are found incidentally during CCTA or DSA examinations or autopsy, and only few are causing related myocardial ischemia or myocardial infarction. Coronary artery anomalies can be classified into three types: anomalous origin, anomalous course and anomalous termination (7). Origin of the left or right main coronary artery from the opposite or non-coronary sinus with anomalous course has an estimated prevalence of 0.1–0.2%. An anomalous origin of the right coronary artery from the left sinus of Valsalva is estimated to be six to ten times more common than an anomalous left coronary artery arising from the right sinus of Valsalva (8). This type of anomalous origin is often accompanied with four types of anomalous course: retroaortic, prepulmonic, septal or subpulmonic, and interarterial (7). In general, the first three types are considered benign, while the interarterial type is considered malignant, with a high risk of sudden cardiac death. It is assumed that dilation of the aorta and pulmonary artery possibly compresses the intramural segment of the coronary artery during exercise (4,8-10), predisposing to myocardial ischemia or myocardial infarction. In addition, coronary blood flow could possibly be limited by sharp angulation of the ostial segment of the anomalous main coronary artery segment during exercise (11).
In children, CCTA allows accurate depiction of coronary artery anomalies with reasonable radiation exposure. CCTA defines the location of the anomalous origin, details of the intramural segment, and the angle between the ostium and proximal segment (12). CCTA is complementary to invasive angiography and can predict difficult selective intubation of the coronary artery when the ostial angle is acute. Further CCTA can provide detailed description of the relationship to extra-vascular structures and distinguish coronary compression by surrounding structures at the lesion site from plaque/thrombosis. CCTA could also reveal Kawasaki disease involving coronary arteries, coronary sclerosis, thrombosis. However, CCTA is more limited in the evaluation of myocardial characteristics.
In complex clinical scenarios, CT and magnetic resonance imaging (MRI) are often complementary. CMR often allows to define the proximal course of an anomalous coronary artery, but is more limited for coronary assessment compared to CCTA (13,14). However, CMR allows to evaluate cardiac structure, function, myocardial perfusion, myocardial viability, tissue characteristics (edema, hyperemia, necrosis, myocardial hemorrhage, fatty infiltration, etc.) and allows to differentiate myocardial infarction from myocarditis and various forms of cardiomyopathy. In this case, CMR confirmed myocardial infarction while excluding fulminant myocarditis and primary cardiomyopathy. LGE was consistent with ischemic damage in the left coronary artery territory. Further, the endocardial distribution of LGE is typical of ischemic disease, while myocarditis would affect the mid- or epicardial layers of the myocardium. CMR also showed MVO and IMH in the basal-lateral, and mid-anteroseptal wall. LGE size, MVO, and IMH are considered to be independent predictors of MACE (15,16), and have prognostic value. The calculated EF (59%) indicated global left ventricular function was being restored although global circumferential strain and longitudinal strain were reduced.
In conclusion, myocardial infarction is rare in children, but definitive determination of the underlying etiology is of great significance for treatment and prognosis. CCTA can non-invasively identify coronary anomalies, including the anomalous origin and course. It can also provide evaluation of myocardial injury (edema, necrosis, MVO, IMH) and has potentially prognostic value. Therefore, CCTA and CMR are complementary and should be used in particular in complex cases for the diagnosis, evaluation and prognosis in children with cardiogenic shock.
Acknowledgments
We would like to thank Professor Paul Schoenhagen at Cleveland Clinic, for his language and copyediting assistance and mentorship during the preparation of this manuscript.
Funding: This study is funded by the commonweal project of Jinhua Municipal Science and Technology Bureau (2018-4-009).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/cdt-20-422
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-422). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this study and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rigollaud JM, Jimenez M, Vallot M, et al. Myocardial infarction in a child with an anomalous left coronary artery arising from the right coronary sinus. Value of echocardiography. Arch Mal Coeur Vaiss 2001;94:499-503. [PubMed]
- Koestenberger M, Nagel B, Gamillscheg A, et al. Myocardial infarction in an adolescent: anomalous origin of the left main coronary artery from the right coronary sinus in association with combined prothrombotic defects. Pediatrics 2007;120:e424-e427. [Crossref] [PubMed]
- Desai A, Patel S, Book W. "Myocardial infarction" in adolescents: do we have the correct diagnosis? Pediatr Cardiol 2005;26:627-31. [Crossref] [PubMed]
- Kannam HC, Satou G, Gandelman G, et al. Anomalous origin of the left main coronary artery from the right sinus of Valsalva with an intramural course identified by transesophageal echocardiography in a 14 year old with acute myocardial infarction. Cardiol Rev 2005;13:219-22. [Crossref] [PubMed]
- Liu XD, Sun CL, Mu SP, et al. Acute myocardial infarction in a child with myocardial bridge. World J Emerg Med 2011;2:70-2. [Crossref] [PubMed]
- Subramanian S, Gaum WE. Acute myocardial infarction caused by transient coronary vasospasm in a child with Kawasaki disease and no coronary aneurysms. Pediatr Cardiol 2010;31:875-7. [Crossref] [PubMed]
- Kim SY, Seo JB, Do KH, et al. Coronary artery anomalies: classification and ECG-gated multi-detector row CT findings with angiographic correlation. Radiographics 2006;26:317-33; discussion 333-4. [Crossref] [PubMed]
- Peñalver JM, Mosca RS, Weitz D, et al. Anomalous aortic origin of coronary arteries from the opposite sinus: a critical appraisal of risk. BMC Cardiovasc Disord 2012;12:83. [Crossref] [PubMed]
- Lee BY. Anomalous right coronary artery from the left coronary sinus with an interarterial course: is it really dangerous?. Korean Circ J 2009;39:175-9. [Crossref] [PubMed]
- Khalighi K, Sharma M, Toor A, et al. Anomalous Left Main Coronary Artery Arising from the Right Sinus of Valsalva in a Young Man Presenting with Recurrent Syncope and Myocardial Infarction. Case Rep Cardiol 2018;2018:9805061.
- Lorenz EC, Mookadam F, Mookadam M, et al. A systematic overview of anomalous coronary anatomy and an examination of the association with sudden cardiac death. Rev Cardiovasc Med 2006;7:205-13. [PubMed]
- Kobrossi S, Saade C, Alajaji W, et al. Anomalous origin of the right coronary artery from the mid-left anterior descending coronary artery: association with acute myocardial infarction. BJR Case Rep 2017;4:20170031. [Crossref] [PubMed]
- Ishii M, Sato Y, Matsumoto N, et al. Acute myocardial infarction in a patient with anomalous origin of the right coronary artery: depiction at whole-heart coronary magnetic resonance angiography and delayed-enhanced imaging. Int J Cardiol 2008;131:e22-e24. [Crossref] [PubMed]
- Lee J, Choe YH, Kim HJ, et al. Magnetic resonance imaging demonstration of anomalous origin of the right coronary artery from the left coronary sinus associated with acute myocardial infarction. J Comput Assist Tomogr 2003;27:289-91. [Crossref] [PubMed]
- Reindl M, Holzknecht M, Tiller C, et al. Impact of infarct location and size on clinical outcome after ST-elevation myocardial infarction treated by primary percutaneous coronary intervention. Int J Cardiol 2020;301:14-20. [Crossref] [PubMed]
- Hamirani YS, Wong A, Kramer CM, et al. Effect of microvascular obstruction and intramyocardial hemorrhage by CMR on LV remodeling and outcomes after myocardial infarction: a systematic review and meta-analysis. JACC Cardiovasc Imaging 2014;7:940-52. [Crossref] [PubMed]


