
This article has an erratum available at: http://dx.doi.org/10.21037/cdt-2023-6 the article has been update on 2023-11-02 at here.
MicroRNA-221 promotes proliferation and migration of pulmonary arterial smooth muscle cells (PASMCs) by targeting tissue inhibitor of metalloproteinases-3 (TIMP3)
Introduction
Pulmonary arterial hypertension (PAH) is a multifactorial disease with a poor prognosis, which can lead to increased right ventricular afterload and ultimately right heart failure and death (1,2). It is well recognized that aberrant proliferation of vascular smooth muscle cells (VSMCs) is a key cellular event in the pathogenesis of many proliferative vascular diseases such as PAH, which results in the narrowing or occlusion of the pulmonary vessels (3). Since current therapies for PAH are primarily vasodilators, novel therapies for PAH are highly needed.
MicroRNAs (miRNAs, miRs) are a class of endogenous, small, non-coding RNAs that negatively regulate gene expression by targeting at the 3'-untranslational regions (UTRs) of mRNAs for gene degradation or translational inhibition (4). MiRNAs have multiple cellular functions including regulation of cell metabolism, development, proliferation and death (5-7). MiR-221 belongs to the miR-221/222 family and has been reported to play an important role in multiple cancer types (8,9). Moreover, miR-221 has been found to regulate essential physiological vascular processes (9-11). In VSMCs, platelet-derived growth factor (PDGF)-induced miR-221 expression leads to the inhibition of several cell cycle regulators such as p27Kip1, p57Kip2 and c-kit (9,10). Nevertheless, potential target genes of miR-221 are still needed to be identified so as to get a further understanding of its function in the pathogenesis of a variety of proliferative vascular diseases.
Tissue inhibitor of metalloproteinases-3 (TIMP3), a member of the tissue inhibitor of metalloproteinases (TIMP) family, was originally characterized as a tumor suppressor and a potent inhibitor of angiogenesis. Through matrix metalloprotein (MMP)-dependent or MMP-independent manner, it regulates multiple physiological functions such as proliferation, apoptosis as well as migration. Low expression of TIMP3 has been found to facilitate tumor growth, angiogenesis, invasion, and metastasis and suppress apoptosis (12-14). In addition, TIMP3 is a newly identified target gene of miR-221 in variety cancer cells such as breast cancer, colorectal cancer and hepatocellular carcinoma (15,16). However, the relationship between TIMP3 and miR-221 in pulmonary arterial smooth muscle cell (PASMC) has not been determined. In this study, we aim to clarify the role of miR-221 on proliferation and migration of SMC originated from pulmonary artery and determined potential target genes involved in this biological function. We present the following study in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/cdt-20-328).
Methods
Animals
This study was performed under a project license (No. IACUC-1803019) granted by institutional ethics board of Nanjing Medical University, in compliance with the guidelines on humane use and care of laboratory animals for biomedical research published by National Institutes of Health (No. 85-23, revised 1996).
Cell culture
The PASMCs were obtained from the pulmonary arteries of male Sprague-Dawley (SD) rats (5 weeks old) by a tissue-sticking method. Briefly, the healthy rats were sacrificed by cervical dislocation. After removal of adventitia and endothelia, pulmonary artery was cut into small pieces and moved to cell culture bottle. The PASMCs were determined by immunostaining of α-smooth muscle actin (α-SMA, Sigma-Aldrich, St. Louis, MO, USA). The basic culture medium consisted of Dulbecco’s modification of Eagle’s medium-F12 (DMEM-F12) supplemented with 5% fetal bovine serum (FBS) while the starvation medium with 1% FBS. Cells between passages 3 and 6 were used for experiments.
Human embryonic kidney 293T cells were purchased from the American Type Culture Collection (ATCC, Washington D.C., USA) and were cultured in DMEM supplemented with 5% FBS.
Cell transfection
Before transfection, PASMCs were starved for 8 hours for cycle synchronization. Lipofectamine 2000 reagent was used to transfect miR-221 mimics (50 nM, Ribobio, Guangzhou, China), miR-221 inhibitors (100 nM, Ribobio, Guangzhou, China) and their negative controls into the different groups according to the instructions. SiRNA-TIMP3 (50 nM, Ribobio, Guangzhou, China) was transfected into PASMCs to deplete TIMP3. SiRNA-TIMP3 sequence was as follows: sense 5'-3' GCUAUC-AGUCCAAACACUATT; anti-sense 5'-3' UAGUGUUUGGACUGAUAGCTT. SiRNA-NC sequence was as follows: sense 5'-3' UUCUCCGAACGUGUCACGUTT, anti-sense 5'-3': ACGUGACACGUUCGGAGAATT.
EdU cell proliferation assay
PASMCs are stimulated by PDGF-BB (20 ng/mL) for 24 hours and then incubated with miR-221 inhibitor, miR-221 mimics and their controls respectively for 24 hours. The proliferation of PASMCs was detected by 5-ethynyl-2'-deoxyuridine (EdU) assay. Briefly, EdU was added to the medium 8 hours before harvest so it was incorporated into replicating DNA. Then cultured cells were washed three times by PBS and fixed with 4% paraformaldehyde for 20 min; 0.2% Triton X-100 was used to permeabilise the nuclear membrane and PBS containing 10% goat serum was used to block the cells for 1 hour at room temperature. PASMCs were then incubated with α-smooth muscle actin antibody (Sigma, St. Louis, MO) at 1:500 overnight. After washing with PBS for three times, PASMCs were stained by Cell-Light™ EdU Apollo®488 in vitro Imaging Kit (Life Technologies, NewYork USA) according to the instructions. Nuclei were stained with 4',6-diamidino-2-phenylindole (DAPI). Finally, the cell images were captured with Nikon eclipse Ti microscope and the number of EdU positive cells was analyzed by Image J software.
Cell migration
Cell migration was determined by a scratch wound assay as previously described (17). Briefly, PASMCs were cultured in six well plates in starvation medium and wounded with a sterile pipette tip to generate a cell-free gap of 1 mm width, and the wound location in the culture dish was marked as described. Cells were photographed to record the wound width at 0 h. After that, cells were treated according to requirement; 24 hours later, photographs will be taken again at the marked wound location for migration measurement.
Quantitative real-time PCR
Total RNA was extracted from cell samples using the miRNeasy Mini Kit (Qiagen, Hilden, Germany). The expression of miR-221 was measured by quantitative real-time PCR according to the manufacturer’s protocol (Ribobio, Guangzhou, China). As an internal control, U6 was used for miRNA template normalization and GADPH was used for protein-coding RNA template normalizations.
Western blot analysis
Proteins were extracted from cultured cells and protein expression levels were determined by western blot analysis. Briefly, equal amounts of protein were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Standard western blot analysis was conducted using TIMP3 antibody (1:500 ProteinTech), and glyceraldehyde-3-phosphate dehydrogenase (GADPH) antibody (1:5,000 dilution; Cell Signaling) as a loading control.
Luciferase assay
A construct in which a fragment of the 3'-UTR of TIMP3 mRNA containing the putative miR-221 binding sequences was cloned into pmiR-RB-Report, which was a firefly luciferase reporter construct (Ribobio, Guangzhou, China). Human embryonic kidney 293T cells (ATCC, Washington D.C., USA) were seeded into 96-well plates and co-transfected with TIMP3-3'UTR-Luc (1 µg) and miR-221 mimic (50 nM) by Lipofectamine 2000. In addition, the construct with mutated fragment of the 3'-UTR of TIMP3 containing disturbed miR-221 binding sequences was used as a negative control. Following 48 hours incubation, luciferase activity was measured on a scintillation counter by a dual-luciferase reporter system (GeneCopoeiaTM, luc-Pair TM Duo-Luciferase Assay kit 2.0, USA).
Monocrotaline (MCT) induced-PAH model
Adult male SD rats purchased from Beijing Weitong Lihua Experimental Animal Limited Liability Company weighting 180 to 220 g were used in this study. Rats were administered with an intraperitoneal injection of MCT (60 mg/kg Sigma-Aldrich) to induce PAH or received vehicle as control. Hemodynamic studies were carried out 28 days after MCT or vehicle injection after which the rats were euthanized for lung tissues collection.
Hemodynamic studies
Rats were anesthetized with 10% chloral hydrate intraperitoneally and ventilated with room air. Right ventricular systolic pressure (RVSP) were then measured in open-chest rats with a pressure-conductance catheter (model 1.4F Millar SPR-671) inserted into the right ventricle. RV pressures were recorded digitally by a signal processor (AD Instruments, USA).
Histological examination
Lung sections were embedded in 4% paraformaldehyde at 4 °C overnight and then were stained with haematoxylin and eosin (H&E). Image J software was used to quantify the medial wall thickness and area. Small arteries ranging from 25 to 100 µm were measured under microscope at ×400 magnification. The medial wall thickness was calculated as previously described. Briefly, the medial thickness (MT) was defined as the distance between the external and internal elastic laminae. The external diameter (ED) and inner diameter (ID) were measured. Percent wall thickness was calculated as (ED − ID)/ED. Percent wall area was calculated as [(total vessel area) − (lumen internal area)]/(total vessel area). Sections were randomly selected and the observers were blinded.
Statistical analyses
Data were expressed as mean ± standard deviation (SD). The statistical significance was assessed by using one-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test for multiple-group comparisons. Differences between two groups were using a paired-sample Student’s t-test. P<0.05 was considered statistically significant.
Results
MiR-221 regulates cell proliferation
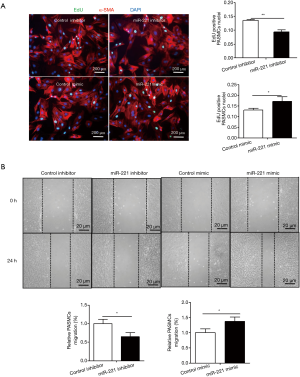
To clarify the effect of miR-221 on PASMCs proliferation, 100 nM miR-221 inhibitor or 50nM miR-221 mimic and their respective control were transfected into PASMCs. EdU assay revealed that miR-221 inhibitor decreased PASMCs proliferation whereas miR-221 mimics increased EdU positive cells (Figure 1A). In addition, migration determined by the scratch wound assay showed that miR-221 inhibitor could inhibit migration while miR-221 mimics was found to promote migration of PASMCs (Figure 1B).
TIMP3 is a target gene of miR-221 in PASMCs
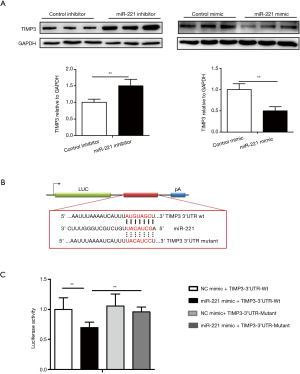
As a member of the tissue inhibitor of TIMP family, TIMP3 can facilitate tumor growth, angiogenesis, invasion, and metastasis and suppress apoptosis. TIMP3 expression was augmented by miR-221 inhibitor and was reduced by miR-221 mimics, indicating that TIMP3 expression was negatively regulated by miR-221 (Figure 2A). As TIMP3 has been proved to be a target gene for miR-221 in tumor cells, in order to verify whether it is the target gene of miR-221 in PASMCs, a pmiR-RB-Report construct containing a fragment of the 3'-UTR of TIMP3 mRNA with the putative miR-221 binding sequences (wt) or with the mutated miR-221 binding sequences (mutant) was constructed (Figure 2B). Co-transfected with pmiR-RB-Report-TIMP3-3'UTR luciferase construct and miR-221 mimics decreased the luciferase activity in PASMCs as compared with mutant construct and NC mimic (Figure 2C).
MiR-221 promotes the proliferation of PASMCs by targeting TIMP3
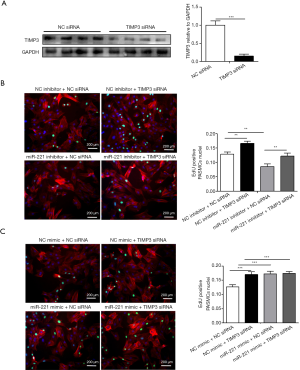
TIMP3 siRNA was designed and synthesized to interfere TIMP3 protein expression and the knockdown effect was evaluated by western blot analysis (Figure 3A). TIMP3 knockdown abolished the inhibitory effect of miR-221 inhibitor on PASMCs proliferation (Figure 3B). In contrast, TIMP3 knockdown failed to provide further enhancive effects on miR-221 mimics-induced PASMCs proliferation (Figure 3C). These data demonstrate that miR-221 promotes PASMCs proliferation through downregulation of TIMP3.
MiR-221 promotes the migration of PASMCs by targeting TIMP3
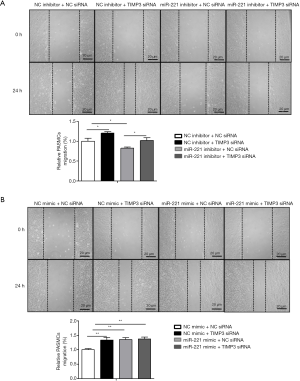
As shown in Figure 4, TIMP3 knockdown abrogated the inhibitory effect of miR-221 inhibitor on PASMCs migration (Figure 4A). However, TIMP3 knockdown did not further aggravate the effects of miR-221 mimics on PASMCs migration (Figure 4B). These data indicate that miR-221 promotes PASMCs migration mainly via targeting TIMP3.
MiR-221 regulates TIMP3 in lung tissues in established PAH model
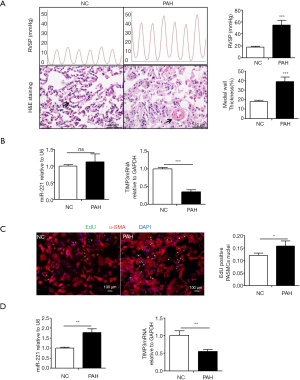
A single injection of MCT can induce PAH, with RVSP values of 58.64±6.47 mmHg in our experiment (Figure 5A). Meanwhile, H&E staining indicated the remodeling of pulmonary vessels, which was evaluated by the arterial MT and area (Figure 5A). All the data above suggested that PASMCs were in a more proliferative state in PAH rats. As miR-221 is highly expressed in VSMCs, especially in cells with high proliferation ratio, we speculated that it might be increased in MCT-treated lung tissues. Total RNA was extracted from lung tissues, miR221 and TIMP3 expression were determined by qRT-PCR. To our surprise, compared with the control group, the expression of miR-221 had no significant difference whereas TIMP3 was decreased dramatically in MCT-treated lung tissues (Figure 5B). To further determine the expression of miR-221 and TIMP3 in PASMCs, cells were obtained from the pulmonary arteries of PAH rats by a tissue-sticking method. As we expected, PASMCs from PAH rats had a higher proliferation rate which was determined by EdU assay (Figure 5C). Besides, qRT-PCR showed that miR-221 was increased while TIMP3 was down-regulated in PASMCs in MCT-treated rats (Figure 5D), suggesting that TIMP3 may be a target of miR-221 in PASMCs of PAH rats.
Discussion
Increased proliferation and migration of VSMCs in small vessels are critical components of pathophysiology in many proliferative cardiovascular diseases, including PAH (1,3). Inhibition of proliferation and migration of VSMC are critical for treating PAH. Increasing evidence shows that miRNAs are involved in the regulation of almost all major cellular functions, such as cell differentiation, apoptosis as well as proliferation and migration (18,19). Here in this study we found that miR-221 could promote PASMCs proliferation and migration, suggesting that inhibition of miR-221 might represent a novel therapy for PAH.
Aberrant expression of miR-221 has been reported in many pathological conditions including cancer, metabolic disease (20) and cardiovascular disease (21). It can also promote the shift of M2-macrophages to a pro-inflammatory function (22). Besides, cell-specific effects of miR-221 have been reported in vessels as well (10). In VSMCs, miR-221 promotes proliferation, migration and decreased apoptosis while in endothelial cells miR-221 has opposite effects (10). In the present study, we found that miR-221 could significantly increase the proliferation and migration of PASMCs. Interestingly, we found that in lung tissues of PAH rats, miR-221 was not changed. However, in PASMCs isolated from PAH rats, miR-221 was significantly increased. These data demonstrated that miR-221 might promote PASMCs proliferation and migration in PAH.
TIMP3 is an extracellular matrix (ECM) bound protein regulating metalloprotease and angiogenic receptors (23). The changes of TIMP3 have been linked to tissue inflammation, fibrosis, and repair (23,24). TIMP3 can protect myocardial infarction by enhancing angiogenesis and inhibiting early proteolysis (25). In heart, TIMP3 deficiency leads to serious myocardial fibrosis but does not result in hypertrophy (26). TIMP3 can also inhibit smooth muscle cells proliferation and migration (27). Of note, we found that miR-221 level was not changed in PAH lung tissues but TIMP3 was decreased, which might be caused by the difference in abundance of these molecules between specific cells types in lung tissues. Importantly, according to the fact that miR-221 was increased while TIMP3 was down-regulated in PASMCs in MCT-treated rats, TIMP3 might be a target gene of miR-221 in PASMCs. Here we also found that TIMP3 inhibit PASMCs proliferation and migration. MiR-221 could negatively regulate TIMP3 in PASMCs. Besides, TIMP3 knockdown abolished the inhibitory effect of miR-221 inhibitor on PASMCs migration and proliferation while TIMP3 knockdown failed to provide additional effects on miR-221 mimics-stimulated PASMCs migration and proliferation. These findings in this study indicated that TIMP3 is a target of miR-221, which enhances PASMCs proliferation and migration mainly through downregulating TIMP3.
Several limitations should be highlighted. Firstly, there was a lack of miR-221 and TIMP3 functional experiment in vivo. The miR-221 or TIMP3 knockout mice will help further clarify their effects in the progress of PAH. Secondly, the pathological changes in PAH locates in the pulmonary distal arteries, however, PASMCs used in our study were obtained from pulmonary trunk. In situ hybridization may be helpful to determine the expression level of miRNA-221 on the small pulmonary lesions. Nevertheless, our study provides direct evidence that miR-221 participates in the progress of PAH by targeting TIMP3, thus providing a potential therapy for PAH.
Conclusions
In conclusion, we found miR-221 enhances PASMCs proliferation and migration mainly by targeting and downregulating TIMP3. These findings suggest that miR-221 and TIMP3 might be two novel therapeutic targets for treating PAH.
Acknowledgments
Funding: This work was supported by the grants from National Natural Science Foundation of China (81730106 and 81670347 to X Li). Dr. X Li is an Associate Fellow at the Collaborative Innovation Center for Cardiovascular Disease Translational Medicine.
Footnote
Reporting Checklist: The authors present the study in accordance with the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/cdt-20-328
Data Sharing Statement: Available at http://dx.doi.org/10.21037/cdt-20-328
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-328). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was performed under a project license (No. IACUC-1803019) granted by institutional ethics board of Nanjing Medical University, in compliance with the guidelines on humane use and care of laboratory animals for biomedical research published by National Institutes of Health (No. 85-23, revised 1996).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McLaughlin VV. Looking to the future: a new decade of pulmonary arterial hypertension therapy. Eur Respir Rev 2011;20:262-9. [Crossref] [PubMed]
- Fine NM, Chen L, Bastiansen PM, et al. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circ Cardiovasc Imaging 2013;6:711-21. [Crossref] [PubMed]
- Morrell NW, Adnot S, Archer SL, et al. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 2009;54:S20-31. [Crossref] [PubMed]
- Farh KK, Grimson A, Jan C, et al. The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. Science 2005;310:1817-21. [Crossref] [PubMed]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136:215-33. [Crossref] [PubMed]
- Li Y, Liang Y, Zhu Y, et al. Noncoding RNAs in Cardiac Hypertrophy. J Cardiovasc Transl Res 2018;11:439-49. [Crossref] [PubMed]
- Xu M, Duan Y, Xiao J. Exercise Improves the Function of Endothelial Cells by MicroRNA. J Cardiovasc Transl Res 2019;12:391-3. [Crossref] [PubMed]
- Zhang C, Zhang J, Hao J, et al. High level of miR-221/222 confers increased cell invasion and poor prognosis in glioma. J Transl Med 2012;10:119. [Crossref] [PubMed]
- Li Y, Liang C, Ma H, et al. miR-221/222 promotes S-phase entry and cellular migration in control of basal-like breast cancer. Molecules 2014;19:7122-37. [Crossref] [PubMed]
- Liu X, Cheng Y, Yang J, et al. Cell-specific effects of miR-221/222 in vessels: molecular mechanism and therapeutic application. J Mol Cell Cardiol 2012;52:245-55. [Crossref] [PubMed]
- Bazan HA, Hatfield SA, O'Malley CB, et al. Acute Loss of miR-221 and miR-222 in the Atherosclerotic Plaque Shoulder Accompanies Plaque Rupture. Stroke 2015;46:3285-7. [Crossref] [PubMed]
- Mavilio M, Marchetti V, Fabrizi M, et al. A Role for Timp3 in Microbiota-Driven Hepatic Steatosis and Metabolic Dysfunction. Cell Rep 2016;16:2269. [Crossref] [PubMed]
- Han XG, Li Y, Mo HM, et al. TIMP3 regulates osteosarcoma cell migration, invasion, and chemotherapeutic resistances. Tumour Biol 2016;37:8857-67. [Crossref] [PubMed]
- Das AM, Bolkestein M, van der Klok T, et al. Tissue inhibitor of metalloproteinase-3 (TIMP3) expression decreases during melanoma progression and inhibits melanoma cell migration. Eur J Cancer 2016;66:34-46. [Crossref] [PubMed]
- Gan R, Yang Y, Yang X, et al. Downregulation of miR-221/222 enhances sensitivity of breast cancer cells to tamoxifen through upregulation of TIMP3. Cancer Gene Ther 2014;21:290-6. [Crossref] [PubMed]
- Zhang W, Peng F, Zhou T, et al. Targeted delivery of chemically modified anti-miR-221 to hepatocellular carcinoma with negatively charged liposomes. Int J Nanomedicine 2015;10:4825-36. [PubMed]
- Kotha J, Zhang C, Longhurst CM, et al. Functional relevance of tetraspanin CD9 in vascular smooth muscle cell injury phenotypes: a novel target for the prevention of neointimal hyperplasia. Atherosclerosis 2009;203:377-86. [Crossref] [PubMed]
- Liu Y, Liu Z, Xie Y, et al. Serum Extracellular Vesicles Retard H9C2 Cell Senescence by Suppressing miR-34a Expression. J Cardiovasc Transl Res 2019;12:45-50. [Crossref] [PubMed]
- Wang L, Lv Y, Li G, et al. MicroRNAs in heart and circulation during physical exercise. J Sport Health Sci 2018;7:433-41. [Crossref] [PubMed]
- Shi C, Wu YY, Wei LQ. MiR-221 affects the proliferation and apoptosis of laryngeal cancer cells through the PI3K/AKT signaling pathway. Eur Rev Med Pharmacol Sci 2020;24:1258-63. [PubMed]
- Abak A, Amini S, Sakhinia E, et al. MicroRNA-221: biogenesis, function and signatures in human cancers. Eur Rev Med Pharmacol Sci 2018;22:3094-117. [PubMed]
- Quero L, Tiaden AN, Hanser E, et al. miR-221-3p Drives the Shift of M2-Macrophages to a Pro-Inflammatory Function by Suppressing JAK3/STAT3 Activation. Front Immunol 2020;10:3087. [Crossref] [PubMed]
- Zhabyeyev P, Das SK, Basu R, et al. TIMP3 deficiency exacerbates iron overload-mediated cardiomyopathy and liver disease. Am J Physiol Heart Circ Physiol 2018;314:H978-H990. [Crossref] [PubMed]
- Stöhr R, Kappel BA, Carnevale D, et al. TIMP3 interplays with apelin to regulate cardiovascular metabolism in hypercholesterolemic mice. Mol Metab 2015;4:741-52. [Crossref] [PubMed]
- Takawale A, Zhang P, Azad A, et al. Myocardial overexpression of TIMP3 after myocardial infarction exerts beneficial effects by promoting angiogenesis and suppressing early proteolysis. Am J Physiol Heart Circ Physiol 2017;313:H224-H236. [Crossref] [PubMed]
- Fan D, Takawale A, Basu R, et al. Differential role of TIMP2 and TIMP3 in cardiac hypertrophy, fibrosis, and diastolic dysfunction. Cardiovasc Res 2014;103:268-80. [Crossref] [PubMed]
- Zhai H, Qi X, Li Z, et al. TIMP-3 suppresses the proliferation and migration of SMCs from the aortic neck of atherosclerotic AAA in rabbits, via decreased MMP-2 and MMP-9 activity, and reduced TNF-α expression. Mol Med Rep 2018;18:2061-7. [PubMed]


