Percutaneous left atrial appendage closure—An alternative strategy for anticoagulation in atrial fibrillation and hereditary hemorrhagic telangiectasia?
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia worldwide and is a major risk factor for cerebral embolic stroke (1-3). Oral anticoagulation (OAC) is highly effective in stroke prevention (1). However, a substantial number of patients are unable to sustain chronic OAC. Among these are patients with hereditary hemorrhagic telangiectasia (HHT).
HHT is an autosomal dominant inherited disease characterized by vascular malformations ranging from small telangiectases in skin and mucosal membranes to large arteriovenous malformations in brain, liver and lungs (4). These patients frequently encounter severe epistaxis and gastrointestinal (GI) bleedings leading to anemia and a substantial decrease in quality of life (QOL). Furthermore, since cerebral arteriovenous malformations (CAVMs) and pulmonary arteriovenous malformations (PAVMs) increase the risk of life threatening bleeding, a relative or absolute contraindication for OAC exists (5).
Most thromboembolic complications in patients with AF arise from the left atrial appendage (LAA) (1,6).
This is the first case series describing the feasibility of percutaneous LAA closure in HHT patients with AF and a high thromboembolic risk (7).
Case reports
Between 2010 and 2012, five consecutive patients (patient number 1-5, 3 males, mean age 71.4±5.0 years) with HHT and high thromboembolic risk AF (median CHA2DS2-VASc score of 4, range, 2-5) (1) received a LAA closure device. The baseline characteristics are described in Table 1.
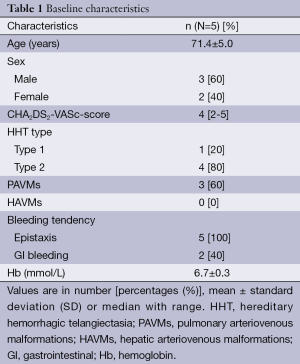
Full table
Before LAA closure, patients 1 to 4 used OAC (CHA2DS2-VASc score 2, 5, 2, 4 respectively). All patients had progressive bleeding problems during this therapy and patient 1 and 2 needed several blood transfusions. Patient 5 (CHA2DS2-VASc score 5) only used aspirin because of a history of severe epistaxis and both an ischemic and hemorrhagic stroke.
LAA closure
Before closure of the LAA, a three dimensional transesophageal echocardiogram (3DTEE) was performed to evaluate the anatomy of the LAA and to exclude pre-existent thrombus formation (Figures 1,2).
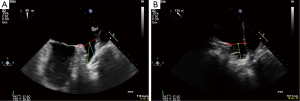
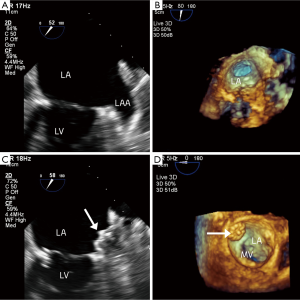
All procedures were performed as written before (3). In all cases the implantation of the LAA closure device (Watchman Left Atrial Appendage Occlusion Device®, Atritech Inc., Plymouth, Minnesota, USA) was acutely successful and there were no peri-procedural complications.
Directly after implantation, patient 1, 3 and 4 continued OAC, patient 5 continued aspirin and patient 2 combined aspirin and clopidogrel.
Follow-up
At three-month follow-up, TEE showed residual flow from LA to the LAA in patient 5 as a sign of incomplete LAA closure. No thromboembolic complications occurred. All patients discontinued OAC because of progressive bleeding; four patients switched to aspirin and patient 5 stopped the aspirin.
At 12-month follow-up (Table 2), one symptomatic episode was documented as a transient ischemic attack (TIA) in patient 1. The MRI showed no signs of ischemia. The TEE 2 months before and after the event showed a small residual flow but the criteria for complete closure were still fulfilled and no thrombus was seen. This patient had a history of smoking, had non-treatable PAVMs and was treated with thalidomide for refractory epistaxis. At the time of the event, the patient used no OAC or antiplatelet therapy. Although the exact cause of the TIA remained unknown, this patient restarted OAC which resulted in severe GI bleeding. Due to recurrent symptomatic AF rhythm surgery with LAA resection was performed.
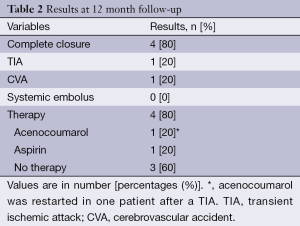
Full table
A minor stroke was reported in patient 5. This patient had a significant stenosis of the carotid artery and embolized PAVMs with a persistent right-to-left shunt (RLS) on contrast echocardiogram. At follow-up, an incomplete LAA closure but no thrombus formation was seen. In the remaining three patients, OAC was withheld without any complications.
Discussion
The treatment of patients with HHT and high thromboembolic risk AF is an increasing problem. Current guidance on the use of antiplatelet and anticoagulant agents in HHT is based on anecdotal evidence and expert opinion (5,8). This leads to insufficient treatment in many patients with a high stroke risk. In the United Kingdom, over 50% of the HHT patients were advised not to use OAC or antiplatelet therapy (8).
To decrease the risk of thromboembolic complications originating from the LAA, a percutaneous LAA closure may be performed safely. At mid-term follow-up, LAA closure proved to be non-inferior with regard to the prevention of stroke, systemic embolism and cardiovascular death in a large study with 707 patients (6).
In this current study, there was a CHA2DS2-VASc score of 4 estimating a yearly stroke risk of 4.0% (9). The thromboembolic complications that occurred during follow-up could be caused by either paradoxical embolization trough PAVMs, carotid artery disease, incomplete closure of the LAA or the use of thalidomide. A PAVM causes a permanent RLS that bypasses the pulmonary capillary filter, which carries the risk of cerebral paradoxical embolization (4,10). An incomplete closure of the LAA may provoke thrombus formation and might allow thrombotic embolization of LAA thrombus through the remaining defect, although current evidence seems contradictory (6,11,12). A small peri-device flow (jet width ≤5 mm) is seen after LAA closure in >30% and the PROTECT-AF trial revealed that this is not associated with an increased thromboembolic risk (12). In this case-series, thalidomide could also have contributed to thrombus formation. Thalidomide is frequently used for the treatment of refractory incapacitating epistaxis in HHT and the thrombotic complications are well known in cancer patients treated with thalidomide (13).
Currently, no guidelines regarding the treatment of patients with an incomplete LAA closure and an absolute contraindication for OAC exist. One report describes the safety of percutaneous LAA closure with another LAA closure device [the Amplatzer cardiac plug (ACP)] in 60 patients (no HHT patients) with a contraindication to OAC (14). After LAA closure, antiplatelet therapy was started without any thromboembolic complications (device related thrombus occurred in 3.5%) (14). Although there is less evidence, the ACP device may be an option for patients in which the LAA anatomy is not suitable for implantation with a Watchmann device (15).
There is no literature on the use of LAA closure without OAC or antiplatelet therapy. However, this therapy seems most important in the first months after implantation when endothelialization of the device is not complete.
Recently, it has been suggested that HHT patients tolerate antiplatelet therapy better than OAC (8). Besides this bridging therapy with OAC might not be necessary after LAA closure, based on the recent ASAP trial (16). Therefore, LAA closure seems especially valuable in HHT patients with intolerance for OAC who otherwise would be treated with antiplatelet therapy alone.
The treatment strategy in patients with both HHT and AF induced high stroke risk remains challenging and no sufficient answer for this specific population has been found. Treatment with OAC may lead to progressive and severe bleeding with a decrease in QOL. However, guidance for the treatment of patients with an incomplete LAA closure in this specific subgroup is lacking. Secondly, other thromboembolic risk factors may exist, especially in HHT.
Based on our current experience, a tailor made approach is necessary in which the choice for OAC or LAA closure should be based on the thromboembolic risk, the presence of visceral arteriovenous malformations and the bleeding tendency of the patient. We recommend to select patients with HHT for percutaneous LAA closure when OAC is not tolerated and after an observational period with and without antiplatelet therapy prior to LAA closure.
In conclusion, percutaneous closure of the LAA may provide an alternative strategy to OAC therapy in HHT patients with AF induced high stroke risk and intolerance for OAC. Future larger studies are needed to reveal the risks and benefits of this therapy in patients with HHT.
Acknowledgements
Disclosure: Dr. Boersma, Dr. Rensing and Dr. Swaans are consultants for Boston Scientific. The Cardiology Department receives proctoring fees from Boston Scientific for training and educational services.
References
- Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation--developed with the special contribution of the European Heart Rhythm Association. Europace 2012;14:1385-413. [PubMed]
- Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004;110:1042-6. [PubMed]
- Swaans MJ, Post MC, Rensing BJ, et al. Percutaneous left atrial appendage closure for stroke prevention in atrial fibrillation. Neth Heart J 2012;20:161-6. [PubMed]
- Velthuis S, Buscarini E, van Gent MW, et al. Grade of pulmonary right-to-left shunt on contrast echocardiography and cerebral complications: a striking association. Chest 2013;144:542-8. [PubMed]
- Faughnan ME, Palda VA, Garcia-Tsao G, et al. International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J Med Genet 2011;48:73-87. [PubMed]
- Reddy VY, Doshi SK, Sievert H, et al. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-Year Follow-up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) Trial. Circulation 2013;127:720-9. [PubMed]
- Velthuis S, Swaans MJ, Mager JJ, et al. Left atrial appendage closure for stroke prevention in patients with atrial fibrillation and hereditary hemorrhagic telangiectasia. Case Rep Cardiol 2012;2012:646505.
- Devlin HL, Hosman AE, Shovlin CL. Antiplatelet and anticoagulant agents in hereditary hemorrhagic telangiectasia. N Engl J Med 2013;368:876-8. [PubMed]
- European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery, Camm AJ, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369-429. [PubMed]
- Begbie ME, Wallace GM, Shovlin CL. Hereditary haemorrhagic telangiectasia (Osler-Weber-Rendu syndrome): a view from the 21st century. Postgrad Med J 2003;79:18-24. [PubMed]
- Lam SC, Bertog S, Sievert H. Incomplete left atrial appendage occlusion and thrombus formation after Watchman implantation treated with anticoagulation followed by further transcatheter closure with a second-generation Amplatzer Cardiac Plug (Amulet device). Catheter Cardiovasc Interv 2015;85:321-7. [PubMed]
- Viles-Gonzalez JF, Kar S, Douglas P, et al. The clinical impact of incomplete left atrial appendage closure with the Watchman Device in patients with atrial fibrillation: a PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) substudy. J Am Coll Cardiol 2012;59:923-9. [PubMed]
- Penaloza A, Vekemans MC, Lambert C, et al. Deep vein thrombosis induced by thalidomide to control epistaxis secondary to hereditary haemorrhagic telangiectasia. Blood Coagul Fibrinolysis 2011;22:616-8. [PubMed]
- Wiebe J, Bertog S, Franke J, et al. Safety of percutaneous left atrial appendage closure with the Amplatzer cardiac plug in patients with atrial fibrillation and contraindications to anticoagulation. Catheter Cardiovasc Interv 2014;83:796-802. [PubMed]
- Park JW, Bethencourt A, Sievert H, et al. Left atrial appendage closure with Amplatzer cardiac plug in atrial fibrillation: initial European experience. Catheter Cardiovasc Interv 2011;77:700-6. [PubMed]
- Reddy VY, Möbius-Winkler S, Miller MA, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J Am Coll Cardiol 2013;61:2551-6. [PubMed]


