A heart team and multi-modality imaging approach to percutaneous closure of a post-myocardial infarction ventricular septal defect
Introduction
Post infarction ventricular septal defect (PI-VSD) is a devastating complication of myocardial infarction (MI) occurring in approximately 0.2% of patients in the early reperfusion era. If the defect is not repaired, the mortality rates are greater than 90%, but operative mortality also remains high at 42.9% (1). Percutaneous closure of a PI-VSD provides an attractive alternative in select patients. However, accurate VSD sizing and anatomical assessment of the defect is crucial for a successful outcome. VSD assessment in previous series has been determined by transthoracic echocardiogram (TTE), transesophageal echocardiogram (TEE), or balloon sizing (2-4). We describe the use and importance of a multidisciplinary team with multi-modality imaging to characterize the VSD and successfully close the defect with a percutaneous approach.
Case presentation
A 70-year-old female presented in cardiogenic shock 7 days after developing fatigue, shortness of breath, and epigastric pain. She was found to have anterior ST elevations on her electrocardiogram and urgent coronary angiography revealed an occluded left anterior descending artery in the mid segment that was treated successfully with a drug eluting stent. No other significant obstructive coronary disease was found. However, she had persistent cardiogenic shock requiring immediate inotrope and pressor support and left ventriculography demonstrated a VSD with significant left-to-right shunting. A TTE was done, which confirmed the PI-VSD, an EF of 40–45%, and no significant valvular pathology.
She was transferred to our institution and was medically stabilized on inotrope and pressor support, but remained in critical condition. A multi-disciplinary team including interventional cardiology, cardiothoracic surgery, cardiovascular critical care, cardiac imaging, and cardiac anesthesiology convened as a heart team to discuss treatment options. It was felt by our surgical colleagues that the risk of surgical treatment of her PI-VSD was high and given her relative hemodynamic stability on inotrope and pressors there was time to consider a percutaneous closure option. A repeat TTE 2 days after presentation showed a mid-septal muscular ventricular septal defect measuring 0.4 cm in diameter (Figure 1, Figure 2A). On day 3 after presentation, Cardiac MRI showed the VSD orifice to be approximately 1.0 cm (Figure 2B) and right heart catheterization revealed a Qp/Qs of 1.8 consistent with a moderate left to right shunt. Cardiac CT imaging on day 9 of presentation, when most of the contraction necrosis had completed, showed a mid VSD orifice of 1.6 cm (Figure 2C).

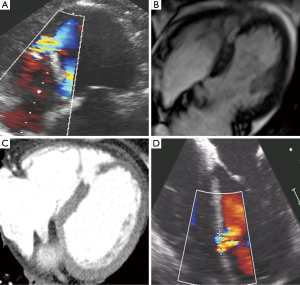
Transcatheter closure of the VSD using the St. Jude PI VSD occluder (St. Jude Medical, Minneapolis, MN, USA) was felt to be the best treatment option for the patient given her risk of open surgical repair, size of the defect, and location with enough rim tissue to anchor the device. Based on CT sizing, a 24-mm Post-MI VSD occluder device was selected. As the device is not FDA-approved for commercial use in the United States, approval for emergent compassionate use of the 24 mm Amplatzer PI-VSD occluder was obtained from the University of North Carolina Institutional Review Board and the patient signed informed consent for the procedure.
The procedure was performed 11 days after presentation in a hybrid operating room with Siemens fluoroscopic imaging, cardiopulmonary bypass on standby, sternotomy equipment readily available in the event emergency sternotomy was required, and with the patient under general anesthesia with continuous TEE guidance. Intra-procedural TEE imaging only measured the VSD to have a 1-cm diameter (Figure 2D). We believed that there were several contributing factors to the differences in PI-VSD measurement among the imaging modalities used. First, the longer the time to imaging likely allowed for greater contraction necrosis to occur and contributed to larger and more accurate sizing of the defect with cardiac CT vs. TTE and cardiac MR. Also, although TTE and TEE have relatively good axial and temporal resolution, the defect size can be underestimated with a less than ideal cross-sectional image alignment through the PI-VSD. With cardiac MR and CT we were able to better establish a cross-sectional plane to measure the largest diameter of the PI-VSD. Also, with regard to comparison of CT vs. MR, cardiac CT has been shown to provide superior spatial resolution than cardiac MR (6). In addition to this, we wanted to avoid balloon sizing as previously described for sizing given the inherent risk of enlarging the PI-VSD. Given the above, we were confident in our CT imaging and used those measurements to guide our selection of the appropriately sized device.
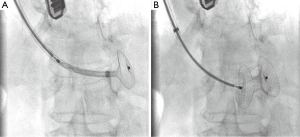
Right femoral artery access was obtained with a six French sheath (Terumo Medical Corporation, Somerset, NJ, USA) and right internal jugular access was obtained with a fourteen French sheath (St. Jude Medical, St. Paul, MN, USA). A six French internal mammary artery (IMA) catheter (Boston Scientific, Marlborough, MA, USA) was brought into the left ventricle and a 300-cm balanced middleweight (BMW) coronary wire (Abbott Vascular, Abbott Park, IL, USA) was used to cross the VSD and positioned into the pulmonary artery. The IMA catheter was exchanged over the BMW wire for a 100-cm glide catheter (Terumo Medical Corporation, Somerset, NJ, USA) to house the coronary wire so as to prevent the coronary wire from slicing into the necrotic myocardial tissue. A 25-cm goose neck snare (EV3 Incorporated, Plymouth, MN, USA) was used to snare the BMW wire in the pulmonary artery and pull it out through the sheath in the internal jugular vein to establish an arteriovenous loop entering through the right femoral artery and exiting the right internal jugular vein. The BMW wire was exchanged for a 260-cm noodle wire (St. Jude Medical, St. Paul, MN, USA). A 12-Fr 45° Torque View sheath with dilator (St. Jude Medical, St. Paul, MN, USA) was advanced from the internal jugular sheath using a catheter kissing technique whereby the Torque View sheath was advanced over the noodle wire as the glide catheter was being retracted over the same wire providing continuous coverage and protection over the noodle wire as it traversed the VSD. The 24-mm Post-MI VSD occluder device was delivered through the delivery sheath across the defect under continuous fluoroscopic and TEE guidance (Figure 3). The device appeared to be in excellent stable position by fluoroscopy and TEE with minimal residual shunt and hemodynamic improvement with immediate improvement in her systolic blood pressure by 20 mmHg and ability to come off inotrope and pressor support quickly (Figure 4, Figure 5A).

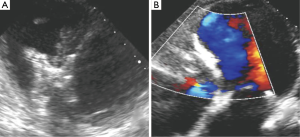
The patient did well after the procedure, with repeat TTE showing trivial residual trans-VSD flow (Figure 5B). She was seen in clinic 2 weeks after her procedure and reported marked improvement in her shortness of breath and fatigue (NYHA I). At 6 months after closure, she is completing cardiac rehabilitation and has returned to work.
Discussion
This case emphasizes the need for a multidisciplinary heart team approach as well as the advantages of multi-modality imaging for selecting the appropriate closure device and for successful deployment of the percutaneous PI-VSD occluder. Cardiac CT provided the highest resolution imaging and best spatial resolution to accurately size the defect and evaluate for sufficient rim tissue to anchor the device. Cardiac CT has not been routinely used for sizing of VSD, but our experience from transcatheter aortic valve replacement (TAVR) has taught us that echocardiography may underestimate the size of an aortic annulus or septal defect, as its measurement in a single plane can measure the defect in short-axis only or in a plane not coaxial to the defect. Accurate sizing is critical, as undersizing could cause serious potential complications such as significant residual shunt as well as device embolization.
In the largest case series described using the Amplatzer device family for PI-VSDs including 53 patients, successful implantation occurred in 89% of patients with a 34% mortality rate at discharge and 13% long term mortality rate for patients that survived to discharge. TEE was used in sizing all VSDs and balloon sizing was used in 49% of patients (3). In this case, cardiac CT provided the critical imaging needed for procedural planning and appropriate device selection, as sizing from echocardiography alone would have led to significant undersizing. Fortunately, the patient was stable enough to allow both cardiac MR and CT in this case at a significant interval from the initial MI to allow for contraction necrosis to occur and therefore more accurate sizing of the PI-VSD and assessment of the surrounding anchoring tissue. In comparison to cardiac MR, cardiac CT has the advantage of greater spatial resolution, faster scan times in more unstable patients, and the ability to scan patients with hardware specific contraindications for MR such as intra-aortic balloon pumps or defibrillators. Although we found that spatial resolution of cardiac CT to be superior to cardiac MR, cardiac MR may be a better option in patients with significant renal dysfunction given the iodinated contrast load of CT and for better tissue characterization when needed. Appropriate sizing of the device and the multi-specialty approach to closure of this VSD helped lead to the successful outcome in this patient (3).
Acknowledgements
There was no federal or industrial support. All authors listed in the authors section have contributed significantly to this manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Arnaoutakis GJ, Zhao Y, George TJ, et al. Surgical repair of ventricular septal defect after myocardial infarction: outcomes from the Society of Thoracic Surgeons National Database. Ann Thorac Surg 2012;94:436-43; discussion 443-4. [Crossref] [PubMed]
- Calvert PA, Cockburn J, Wynne D, et al. Percutaneous closure of postinfarction ventricular septal defect: in-hospital outcomes and long-term follow-up of UK experience. Circulation 2014;129:2395-402. [Crossref] [PubMed]
- Maltais S, Ibrahim R, Basmadjian AJ, et al. Postinfarction ventricular septal defects: towards a new treatment algorithm? Ann Thorac Surg 2009;87:687-92. [Crossref] [PubMed]
- Assenza GE, McElhinney DB, Valente AM, et al. Transcatheter closure of post-myocardial infarction ventricular septal rupture. Circ Cardiovasc Interv 2013;6:59-67. [Crossref] [PubMed]
- Iyer S, Bauer T, Yeung M, et al. Pre-VSD occluder TTE: 4-chamber view with color flow Doppler showing the PI-VSD. Asvide 2016;3:084. Available online: http://www.asvide.com/articles/839
- Crean A. Cardiovascular MR and CT in congenital heart disease. Heart 2007;93:1637-47. [Crossref] [PubMed]
- Iyer S, Bauer T, Yeung M, et al. Post-VSD occluder TEE: transgastric short axis view with color flow Doppler showing minimal residual shunt after successful implantation of occluder device. Asvide 2016;3:085. Available online: http://www.asvide.com/articles/840


