Cardiocutaneous syndrome (Naxos disease) in a Bangladeshi boy
Introduction
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a genetic cardiomyopathy that involves primarily the right ventricle (RV), but also the left, or both ventricles (1). In fact, biventricular and left-dominant forms of the disease are increasingly recognized. ARVC is an important cause of ventricular arrhythmia in children and young adults. In 30–50% of cases of ARVC, a positive family history is present, and the disease is usually inherited in an autosomal dominant pattern with variable penetrance and expressivity (2). Rarely, ARVC can be transmitted in an autosomal recessive manner, usually accompanied by cutaneous manifestations; Naxos disease and Carvajal syndrome are the classical examples of these cardiocutaneous syndromes (2). Here, a case of Naxos disease has been described in a young Bangladeshi boy who presented with ventricular tachycardia (VT) and heart failure.
Case presentation
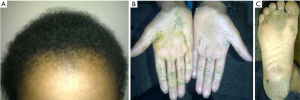
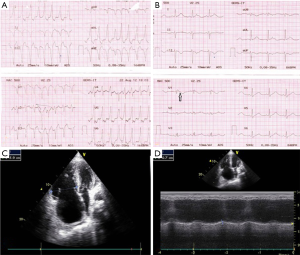
A 10-year-old boy who was the only child of his parents without consanguinity presented with episodic palpitation, progressive breathlessness and swelling of the body. Physical examination revealed wooly hair and keratoderma affecting the palms and soles (Figure 1). His pulse was 110/min, regular, blood pressure 90/70 mmHg, dependent edema, jugular venous pressure (JVP) raised, pansystolic murmur in tricuspid area and hepatomegaly. Previous records revealed admission with VT treated with defibrillation (Figure 2A). Resting ECG showed incomplete right bundle branch block (RBBB) with epsilon wave (Figure 2B). Echocardiography revealed RV cardiomyopathy characterized by RV enlargement, regional hypokinesia, aneurysmal dilatation, impaired systolic function, and moderate tricuspid regurgitation (Figure 2C,D). A diagnosis of Naxos disease was made. He was treated with diuretics, ramipril and sotalol only to give temporary relief. Automated implantable cardioverter defibrillator (AICD) was thought of, but could not be afforded. Nine months after the diagnosis, while staying in his village home, he had sudden cardiac death.
Discussion
Naxos disease is a rare autosomal recessive form of ARVC with a cutaneous phenotype, characterized by woolly hair and palmoplantar keratoderma (3). The disease is most prevalent in the Greek island of Naxos with a prevalence of 1:1,000; however, it has also been reported from Turkey, Israel, Saudi Arabia, India, and Argentina (4). Naxos disease is caused by genes encoding the adhesion molecules, namely, plakoglobin and desmoplakin resulting in weakening and disruption of desmosomes and adherens junctions of myocardium and epidermis predominantly (4). Progressive apoptosis of cardiac myocytes and subsequent fibrofatty replacement provides the anatomic basis of progressive cardiac failure, arrhythmia and sudden death. A similar form inherited cardiocutaneous syndrome but predominantly affecting the left ventricle (LV) constitute the Carvajal syndrome.
The cardinal features of Naxos disease, i.e., ARVC and keratoderma, do not manifest simultaneously; rather they appear at different stages of life. The wooly hair appears at birth, whereas palmoplantar keratoderma develops during the first year of life when the infant starts using the hands and feet (5). The cardiomyopathy in Naxos disease usually presents by adolescence with arrhythmia manifested as syncope and/or VT of left bundle branch block (LBBB) morphology, whereas that in Carvajal syndrome presents at earlier ages of life with heart failure. The case presented here had documented VT, as well as, features of heart failure.
The diagnosis and management of ARVC are at present in evolution; the recently published Revised 2010 Task Force Criteria (TFC) (6) for diagnosis and the International Task Force consensus statement (7) for treatment of ARVC will hopefully bring about uniformity in recognition and management of Naxos disease as well. Currently, no gold standard to establish or exclude the diagnosis of ARVC exists. The revised TFC assign the findings into 6 categories: (I) global and/or regional myocardial dysfunction and structural abnormalities; (II) histological characterization; (III) repolarization abnormalities on 12-lead surface ECG; (IV) depolarization abnormalities on 12-lead surface ECG; (V) arrhythmias; and (VI) family history and genetics; definite diagnosis requires 2 major criteria, 1 major and 2 minor criteria, or 4 minor criteria from different categories (6). For a suspected case, comprehensive non-invasive evaluation should include a thorough clinical history and examination, pedigree analysis, 12-lead surface ECG, transthoracic echocardiography (TTE) with detailed assessment of the RV, cardiac magnetic resonance (CMR), stress testing in order to induce arrhythmias, and Holter ECG monitoring (1). ECG (abnormal in >50% cases) shows T wave inversion in V1 to V3, QRS duration >110 ms in V1 to V3, and epsilon wave (8). TTE features include RV enlargement/dilatation, aneurysm formation, and global or regional hypokinesia mainly in the subtricuspid region, RV outflow tract and RV apex (9). CMR as the non-invasive tool of choice evaluates cardiac morphology, function, and tissue characterization; intra-myocardial fatty infiltration appears as an area of high signal intensity on T1 weighted images (10). Electrophysiological studies are not included in the diagnostic criteria, but may be important for differential diagnosis including RV outflow tract tachycardia. Contrast echocardiography, RV angiography, endomyocardial biopsy (high specificity but low sensitivity, potentially hazardous) and genetic testing may be indicated in selected cases. Because of unavailability, CMR was not done in the index case.
Young age, malignant family history, QRS dispersion ≥40 ms, T wave inversion beyond V1, LV involvement, VT, syncope or previous cardiac arrest are considered as the major determinants of adverse prognosis and impending sudden death. The primary objective of treatment is the prevention of sudden cardiac death. Strenuous exercise should be avoided (class I) (7). Beta blockers in general and sotalol and amiodarone in symptomatic patients are used. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta blockers and diuretics are indicated in heart failure. Oral anticoagulants apply for secondary prevention in intracavitary thrombus or thromboembolism (class I) (7). AICD is recommended for ≥1 episodes of haemodynamically unstable, sustained VT or ventricular fibrillation, or for severe systolic dysfunction (class I) (7). Radiofrequency ablation is indicated in incessant VT or VT requiring frequent AICD shocks (7). Heart transplantation remains for end-stage disease. First-degree relatives are screened with 12-lead ECG, signal-averaged ECG and CMR (11). Genetic counseling applies to future pregnancy.
The case presented here was treated pharmacologically. AICD was indicated, and might prevent sudden death from malignant ventricular arrhythmia. But because of high cost, the device could not be provided. Actually, like other developing countries, this may be an important limiting factor on the way of ensuring the state-of-the-art management strategy for the victims of Naxos disease, and ARVC as well.
Conclusions
Naxos disease, though rare, may affect populations other than the known ones. The diagnosis in presence of the telltale signs of ARVC and palmoplantar keratoderma is usually straightforward. Physicians should have appropriate preparedness to identify and diagnose this rare entity in time, and provide effective treatment including AICD to lessen the morbidity and mortality of the affected persons.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the parent for publication of this case report and any accompanying images.
References
- Pinamonti B, Brun F, Mestroni L, et al. Arrhythmogenic right ventricular cardiomyopathy: From genetics to diagnostic and therapeutic challenges. World J Cardiol 2014;6:1234-44. [Crossref] [PubMed]
- Protonotarios N, Tsatsopoulou A. Naxos disease and Carvajal syndrome: cardiocutaneous disorders that highlight the pathogenesis and broaden the spectrum of arrhythmogenic right ventricular cardiomyopathy. Cardiovasc Pathol 2004;13:185-94. [Crossref] [PubMed]
- Protonotarios N, Tsatsopoulou A. Naxos disease: cardiocutaneous syndrome due to cell adhesion defect. Orphanet J Rare Dis 2006;1:4. [Crossref] [PubMed]
- Baykan A, Olgar Ş, Argun M, et al. Different clinical presentations of Naxos disease and Carvajal syndrome: Case series from a single tertiary center and review of the literature. Anatol J Cardiol 2015;15:404-8. [Crossref] [PubMed]
- Coonar AS, Protonotarios N, Tsatsopoulou A, et al. Gene for arrhythmogenic right ventricular cardiomyopathy with diffuse nonepidermolytic palmoplantar keratoderma and woolly hair (Naxos disease) maps to 17q21. Circulation 1998;97:2049-58. [Crossref] [PubMed]
- Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J 2010;31:806-14. [Crossref] [PubMed]
- Corrado D, Wichter T, Link MS, et al. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Circulation 2015;132:441-53. [Crossref] [PubMed]
- Haugaa KH, Haland TF, Leren IS, et al. Arrhythmogenic right ventricular cardiomyopathy, clinical manifestations, and diagnosis. Europace 2016;18:965-72. [PubMed]
- Baran A, Nanda NC, Falkoff M, et al. Two-dimensional echocardiographic detection of arrhythmogenic right ventricular dysplasia. Am Heart J 1982;103:1066-7. [Crossref] [PubMed]
- te Riele AS, Tandri H, Bluemke DA. Arrhythmogenic right ventricular cardiomyopathy (ARVC): cardiovascular magnetic resonance update. J Cardiovasc Magn Reson 2014;16:50. [Crossref] [PubMed]
- Smith W. Members of CSANZ Cardiovascular Genetics Working Group. Guidelines for the diagnosis and management of arrhythmogenic right ventricular cardiomyopathy. Heart Lung Circ 2011;20:757-60. [Crossref] [PubMed]


